Dancing molecules drive repair in lab-grown human spinal cords
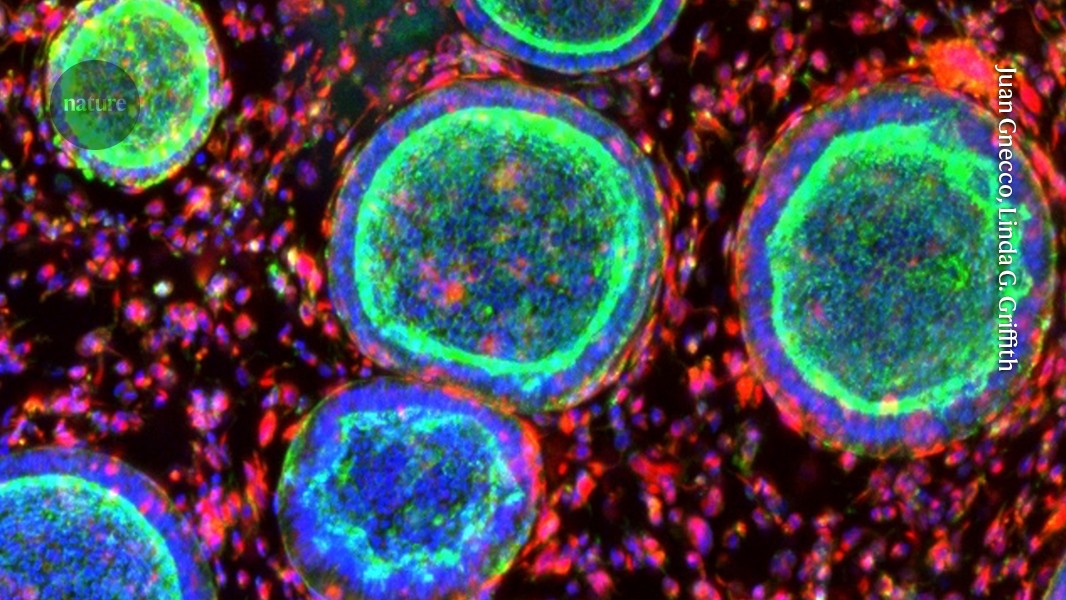
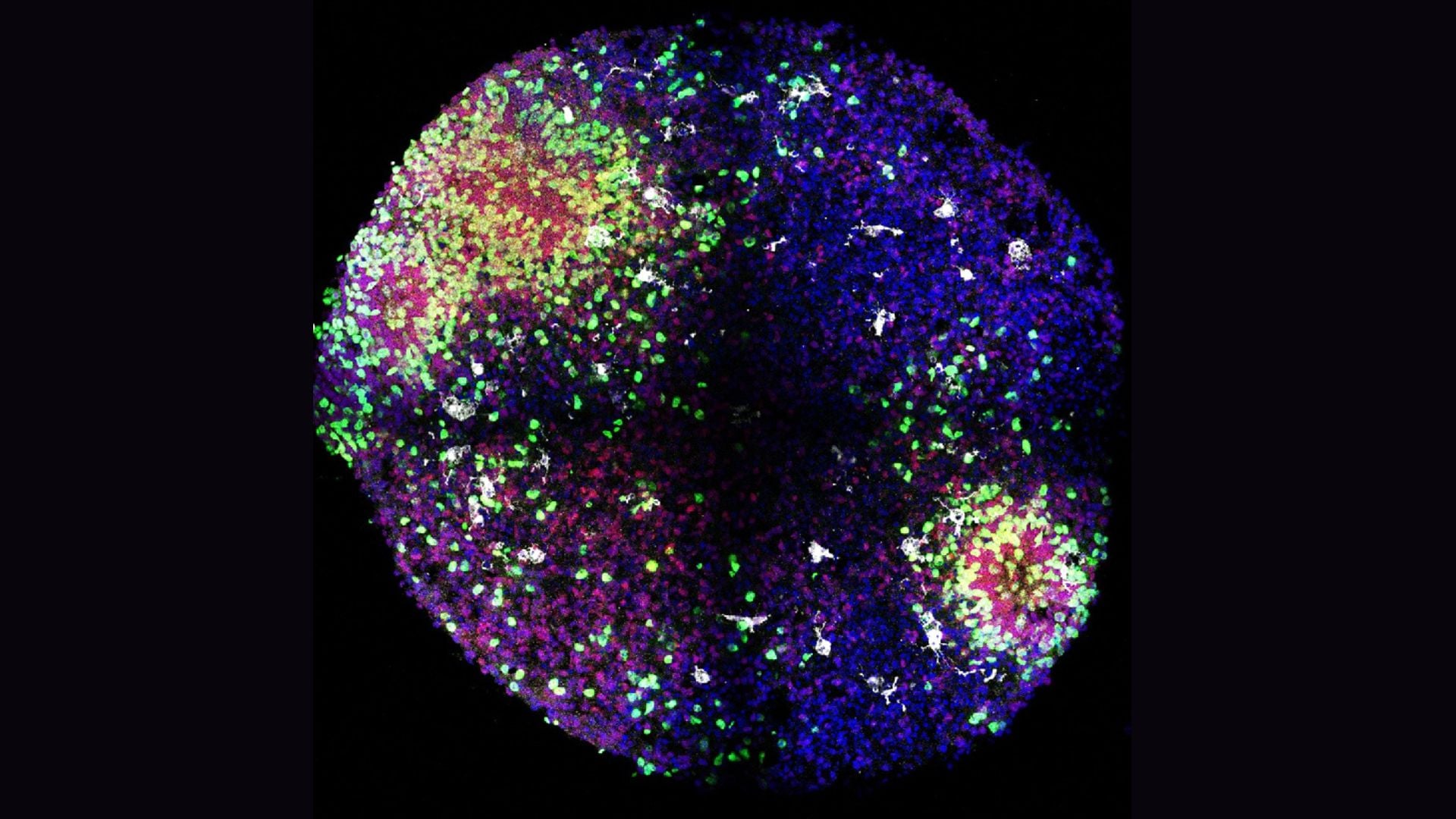
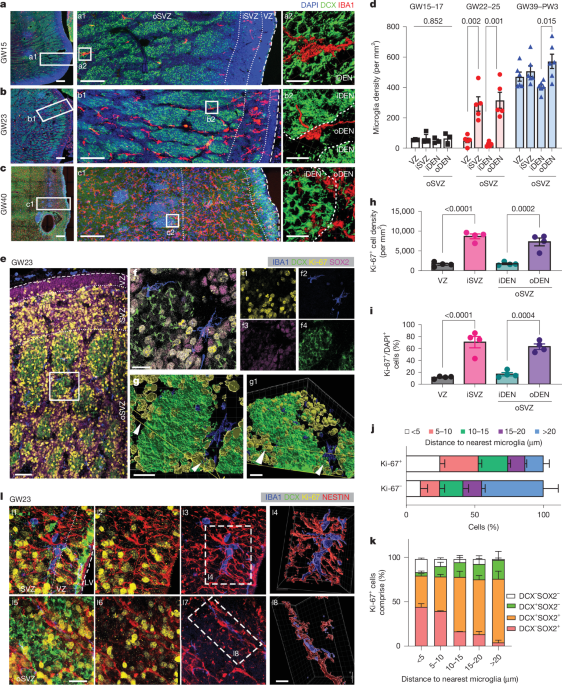
Northwestern researchers grew 3-millimeter-wide human spinal cord organoids from iPSCs, injured them in two ways, and treated them with IKVAV-PA 'dancing' supramolecular peptides that form a scaffold and promote axon regrowth. The treated tissues showed reduced glial scar and inflammation and enhanced neurite growth, aligning with prior mouse data and suggesting potential for human therapies—though clinical use is years away. The work was published in Nature Biomedical Engineering.