Biotechnology News
The latest biotechnology stories, summarized by AI
Featured Biotechnology Stories

ImmunityBio Pursues FDA Path to Resubmission for ANKTIVA in Papillary NMIBC
ImmunityBio announced a productive Type B End‑of‑Phase meeting with the FDA regarding its supplemental Biologics License Application for ANKTIVA plus BCG in BCG‑unresponsive papillary NMIBC. The FDA asked for additional information (not new trials) to support a potential resubmission, which ImmunityBio will provide within 30 days. Long‑term QUILT‑3.032 data in 80 patients show about 96% bladder cancer‑specific survival at 36 months and high cystectomy‑free survival (roughly 82–93% at 12–36 months), underscoring a bladder‐sparing, chemo‑free approach. ANKTIVA is already approved for CIS in the US, UK, and Saudi Arabia, with EU conditional status for CIS and ongoing EMA discussions to extend labeling to papillary disease if US approval is achieved.

More Top Stories
Rocket Pharmaceuticals' Gene Therapy Trial for Danon Disease Faces FDA Hold After Patient Death
Business Wire•9 months ago
More Biotechnology Stories
Radiopharmaceutical Growth Stalled by Talent Shortage
The rise of radiopharmaceuticals, a new cancer treatment that targets cancer cells with radioactive isotopes, is facing a significant talent shortage. This innovative approach has attracted numerous biotech startups and substantial investments, with several companies being acquired for over $1 billion. However, the industry's growth is threatened by a lack of skilled professionals needed to develop and implement these therapies.

Summit Therapeutics' Stock Skyrockets After Cancer Drug Outperforms Keytruda in Phase 3 Trial
Summit Therapeutics' stock surged after the company announced that its cancer drug outperformed Keytruda in a Phase 3 trial, just ahead of a major cancer conference in Chicago.

Intellia Withdraws from Hemophilia Collaboration with Regeneron
Intellia has exited a partnership with Regeneron to develop a factor IX gene editing therapy for hemophilia B, opting out of a deal that was signed in 2020. Despite this, Intellia remains eligible for up to $320 million in future milestones and still holds a 35% stake in a factor XIII insertion program for hemophilia A.

"AI's Evolution in Biology: From Black-Box Data to Understanding Life"
The convergence of AI and biology is expected to lead to a new era of "Biology 2.0," with AI breakthroughs transforming biology into a programmable, predictable, and replicable engineering discipline. The transition from traditional "white-box" data to novel "black-box" data modalities developed alongside computational methods is anticipated to drive significant future breakthroughs. The release of ChatGPT by OpenAI has brought AI into the global spotlight, sparking frenetic optimism around AI in the biological sciences and raising hopes for transformative advancements.

"Latigo Secures $135M for Non-Opioid Pain Medicine, Chasing Vertex Success"
Latigo Biotherapeutics, a non-opioid drug developer, has emerged with a $135 million Series A funding after years of development, targeting the same Nav1.8 as Vertex Pharmaceuticals. The company aims to address pain management without the risk of addiction by focusing on the peripheral nervous system.

"Advancements in Bluebird Gene Therapies, Vertex's Pain Drug, and ADC FOMO"
Bluebird Bio's focus on selling gene therapies, such as Lyfgenia for sickle cell disease, Zynteglo for beta thalassemia, and Skysona for cerebral adrenoleukodystrophy, without other marketed products, makes it a unique player in the gene therapy market, raising questions about the profitability of gene therapies as a business. The company's approach and success over the next year may provide insights into the potential for gene therapies to become a profitable venture.

"Doudna's Aclaris CEO Steps Down, Secures $114M Liver Disorder Deal with Genevant"
Jennifer Doudna's lab published a study detailing how antibody fragments can target virus-like particles with gene editing machinery to T cells, co-founding genetic medicine delivery startup Azalea Therapeutics, which has raised $10 million.

"Billionaire-Backed Biomedical Institute in Cambridge Accelerates Drug Discovery"
A group of billionaires has unveiled their support for a new biomedical research institute, Arena BioWorks, which aims to emulate the success of Bell Labs and lead new research initiatives and create for-profit biotech companies seamlessly under one roof. The institute promises a quicker translational research model, has the backing of big names, and plans to put scientists in the driver’s seat with a footprint in Kendall Square in Cambridge, MA.

CRISPR Revolutionizes CAR-T Pipeline, Expands into Autoimmune Disease Amidst Exa-cel Decision
CRISPR Therapeutics is making changes to its allogeneic CAR-T cell therapy pipeline by cutting two programs, CTX110 and CTX130, and prioritizing next-generation candidates with potential for improved clinical profiles. The company is also expanding into a new indication, autoimmune disease, while awaiting an FDA decision on its CRISPR-edited therapy exa-cel. Patients treated with CTX110 and CTX130 will be transitioned to long-term follow-up programs.
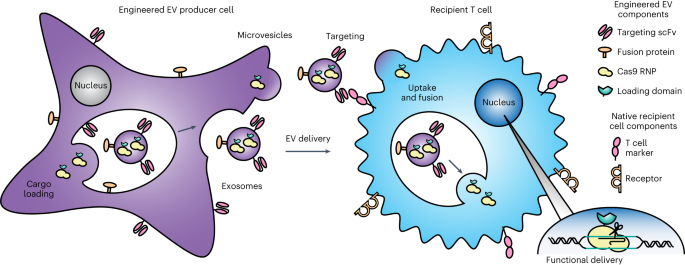
"Enhancing Targeted Biologic Delivery to T Cells through Genetically Encoded Extracellular Vesicles"
Scientists have developed a method to genetically encode multiple functionalities into extracellular vesicles (EVs) for targeted delivery of biologics to T cells. EVs, such as exosomes, have shown promise as vehicles for therapeutic cargo delivery, but their targeting capabilities have been limited. By engineering EVs to express specific ligands or antibodies on their surface, researchers were able to enhance their binding and uptake by T cells. This approach could potentially improve the efficacy of T cell-based therapies and expand their applications in treating various diseases.
