CRISPR Innovation Promises New Era in Genetic Disease Therapy
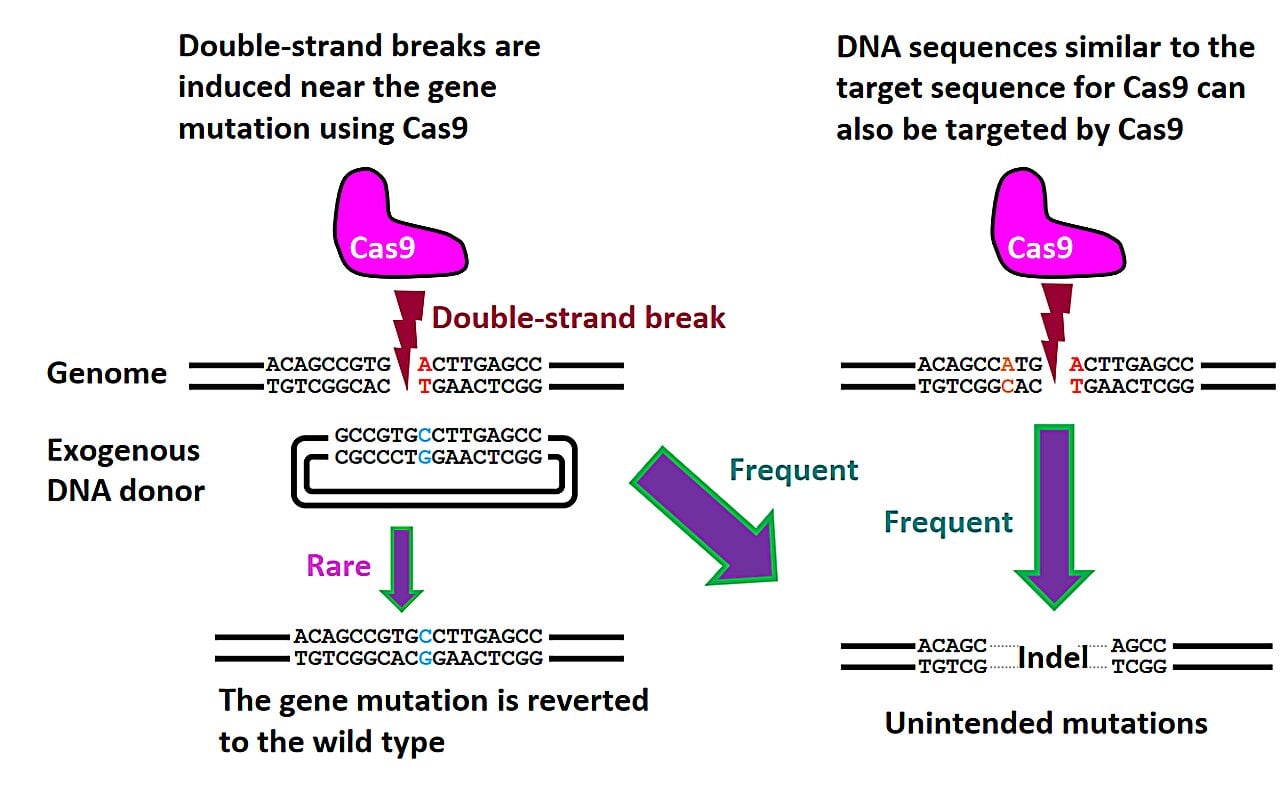
Researchers at UNSW Sydney have developed a safer CRISPR-based epigenetic editing technique that can switch genes on and off without cutting DNA, offering promising new treatments for genetic diseases like Sickle Cell by reactivating silenced genes through removal of methyl groups, potentially reducing risks associated with traditional gene editing.