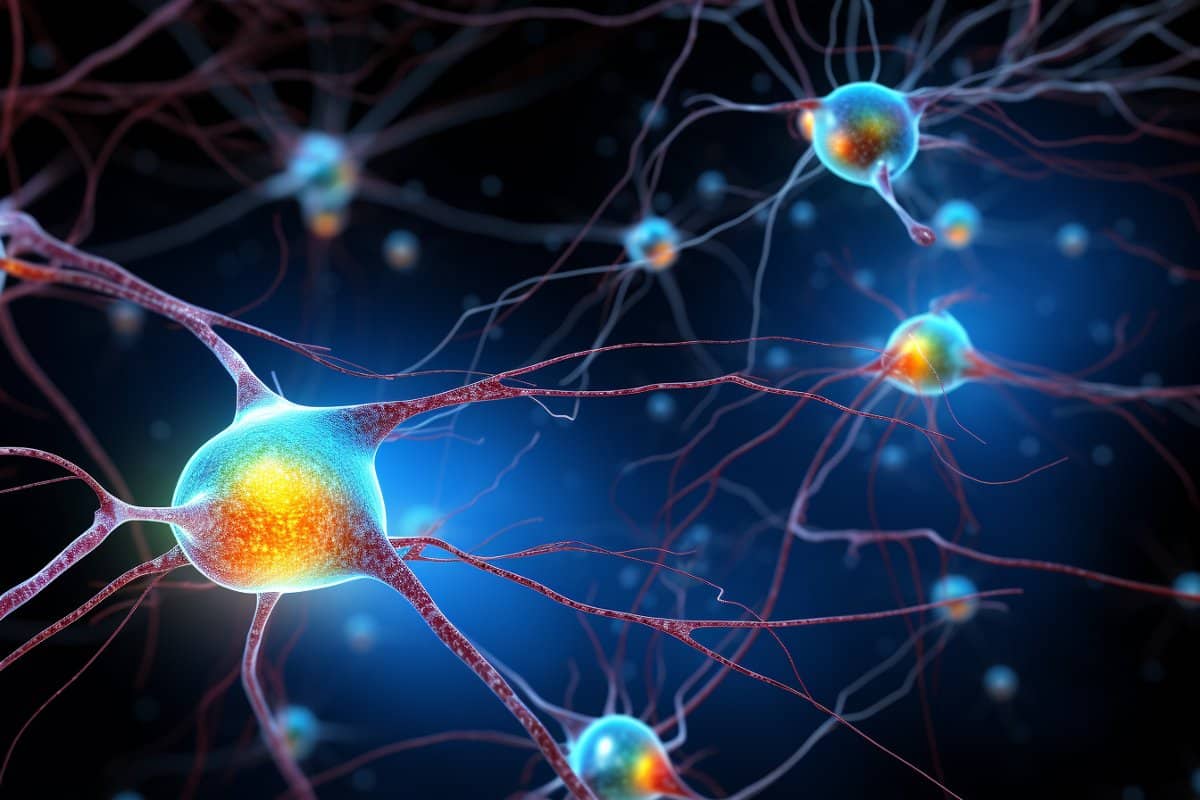
Epigenetic Techniques to Control Memory Activation in Neurons
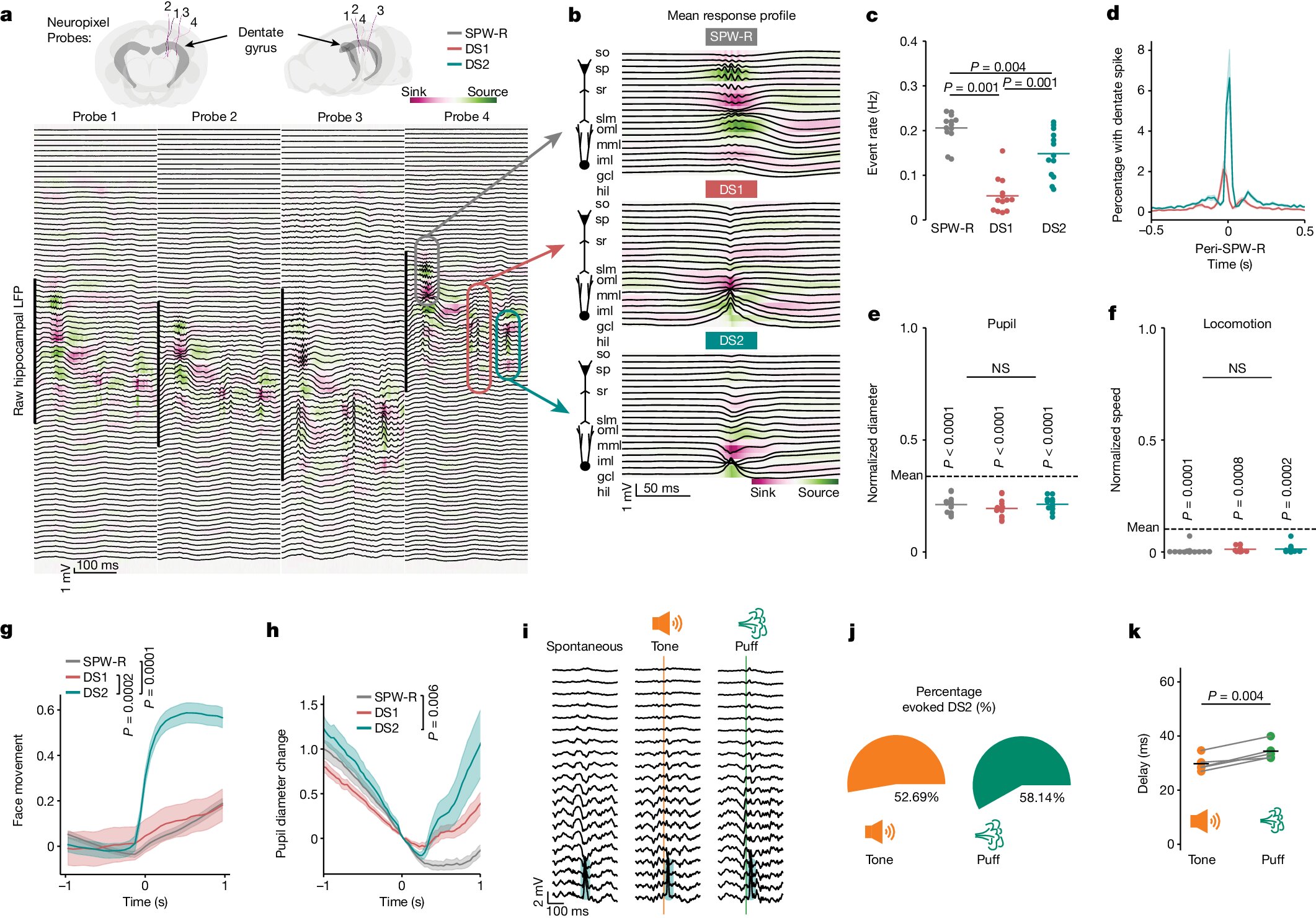
This study developed cell-type- and locus-specific epigenetic editing tools using CRISPR-dCas9 to modulate memory expression in engram cells, demonstrating that targeted epigenetic modifications at the Arc gene locus can bidirectionally regulate memory formation and retention in mice, with effects that are reversible and applicable beyond initial memory consolidation.