Antibody Strategy Targets Epstein-Barr Virus to Shield Transplant Patients
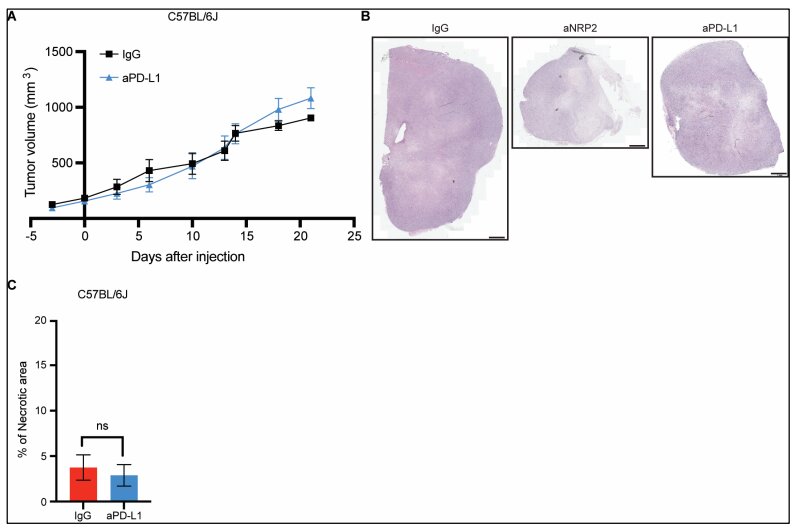
Fred Hutch researchers produced fully human monoclonal antibodies against EBV by targeting its gp350 and gp42 proteins. In humanized mice, one antibody completely prevented EBV infection and another offered partial protection, outlining a pathway to prevent EBV reactivation and PTLD in transplant patients. The team aims to advance safety testing in healthy volunteers followed by trials in immunocompromised individuals, with IP protection filed for the antibodies.