Lab Grows Functional Hair Follicles, Advancing Hair-Loss Treatments
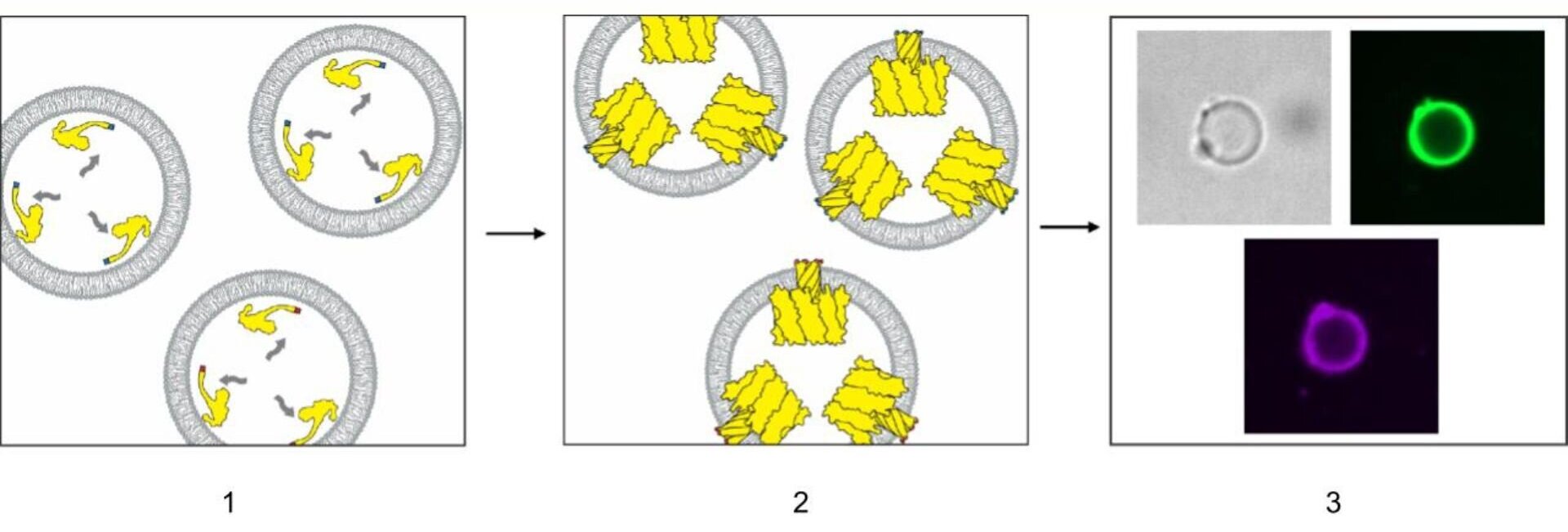
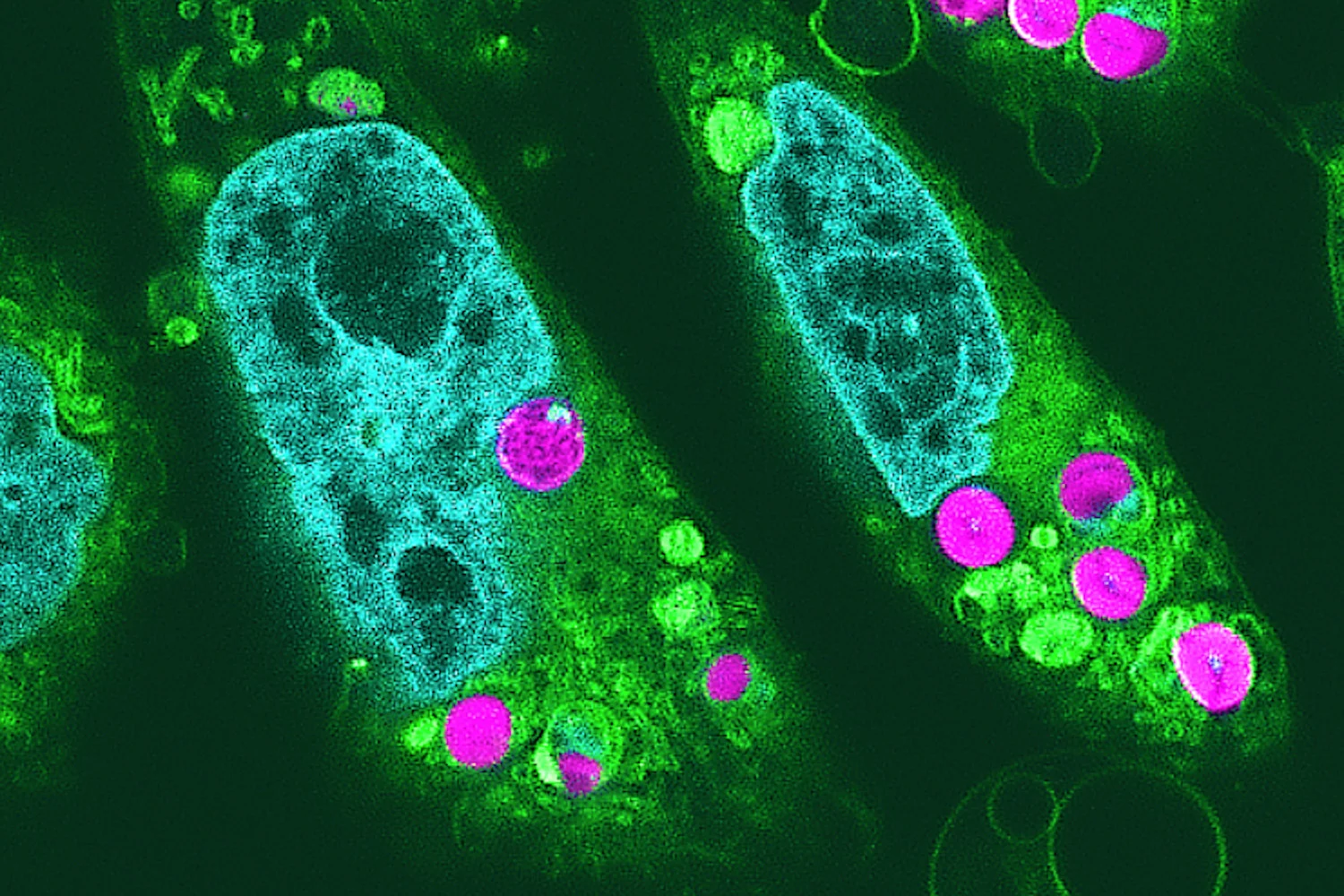
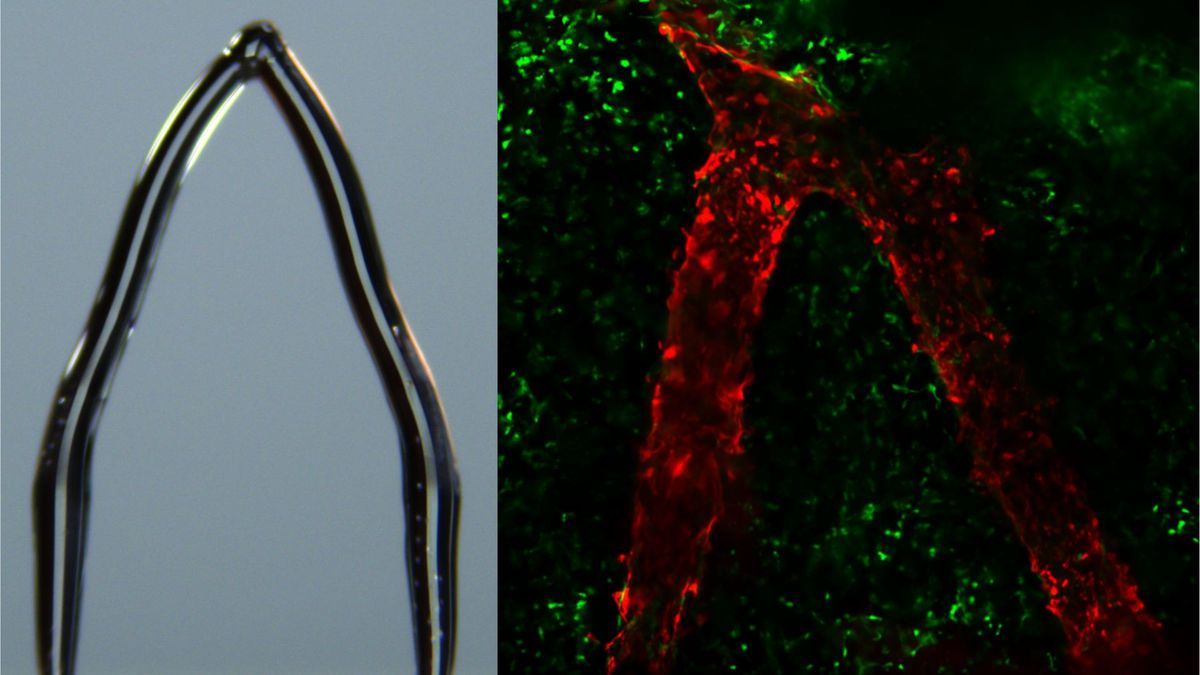
Scientists have grown fully developed, functional hair follicles in the lab by adding a third cell type—accessory mesenchymal cells—alongside epithelial stem cells and dermal papilla cells. This three-cell recipe enables follicles to mature and cycle like natural hair, with potential implications for hair restoration and broader regenerative medicine, though translating the approach to humans remains a key challenge.