Breakthrough in Artificial Cells: Self-Sustained Protein Transport Achieved
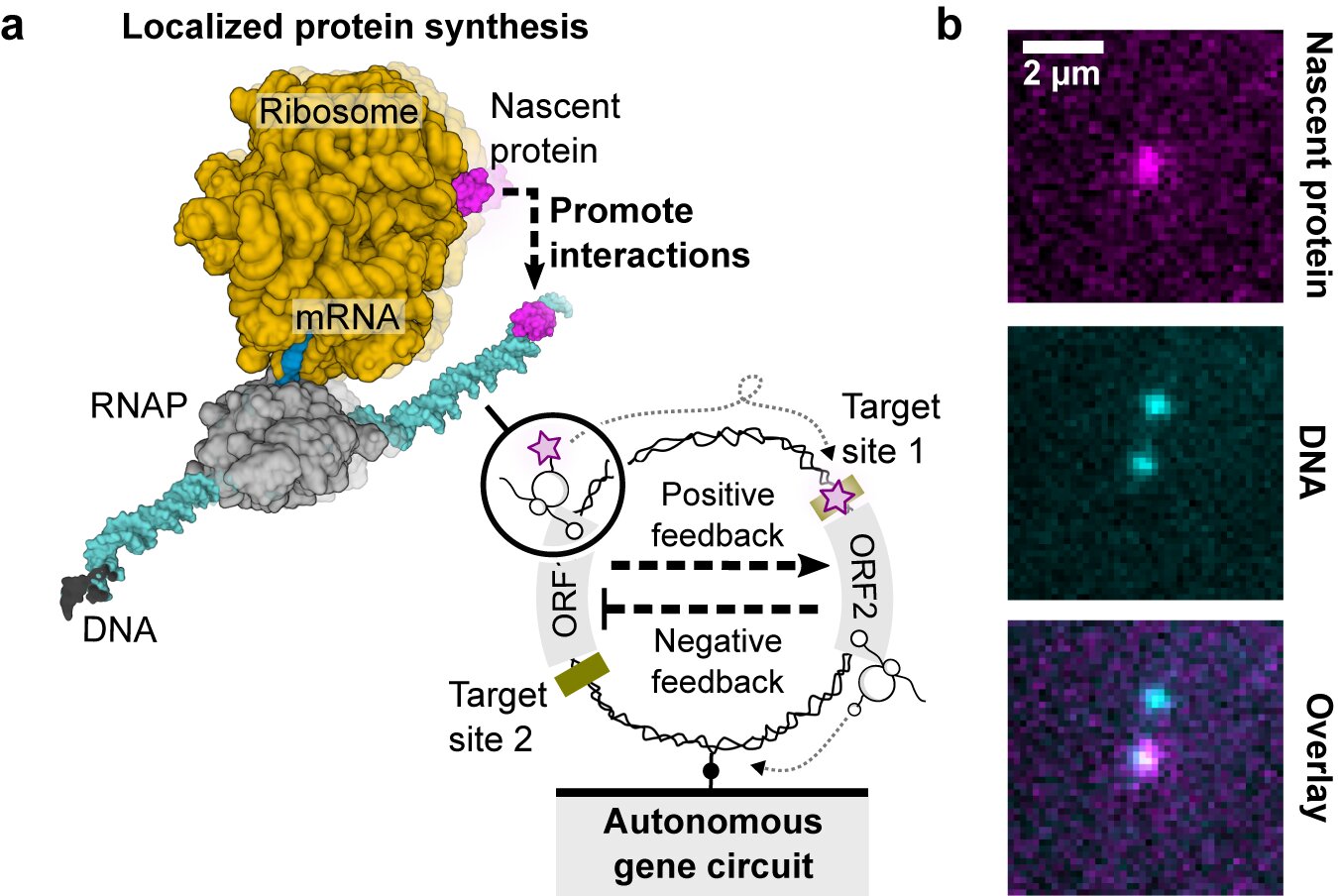
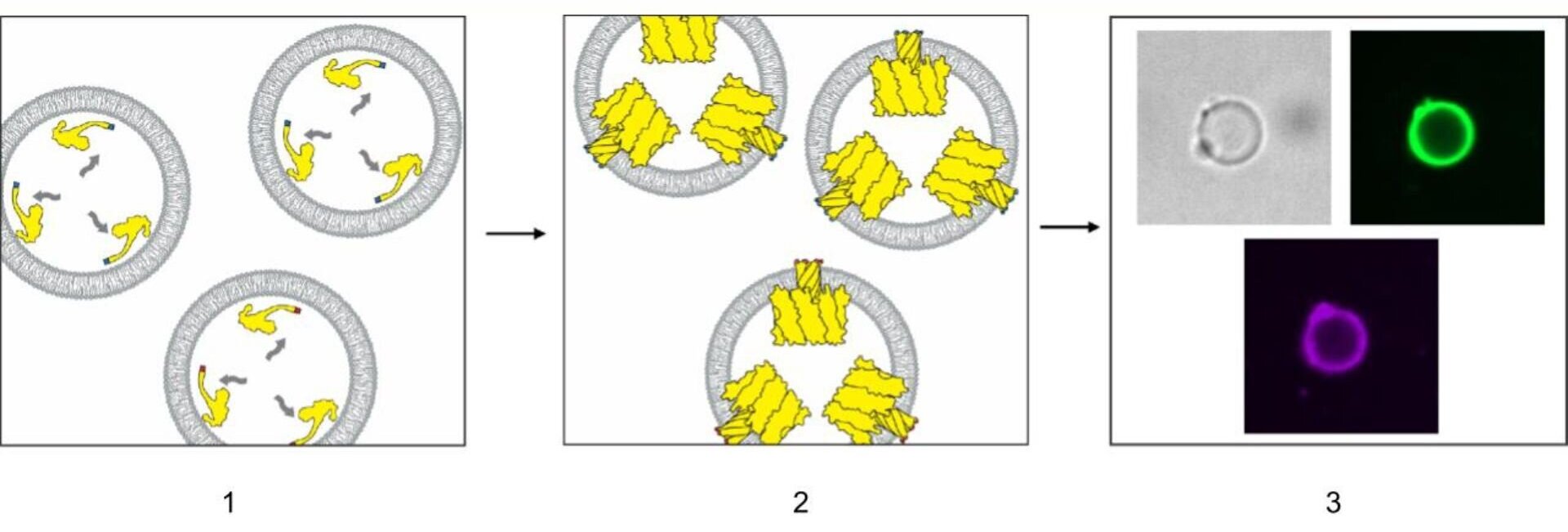
Scientists have developed a method for artificial cells to autonomously modify their membranes, enabling protein transport and tissue assembly without complex external modifications. This breakthrough, using α-hemolysin, could advance tissue engineering and drug delivery by allowing artificial cells to interact with their environment and form tissue-like structures. The study highlights the potential for creating more complex artificial tissues and improving drug delivery systems.