Fecal Transplants Boost Immunotherapy Responses and Cut Side Effects in Cancer Trials
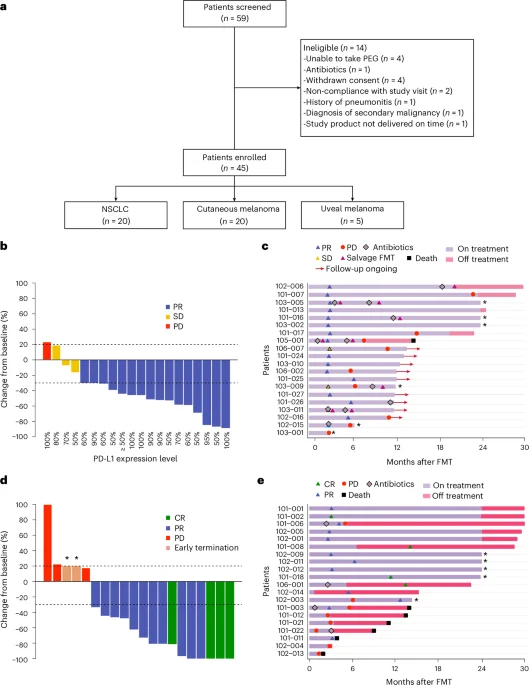
Two Nature Medicine studies show that fecal microbiota transplantation (FMT) capsules can both reduce immunotherapy-related toxicity in kidney cancer and improve response rates in lung cancer and melanoma—80% of lung cancer patients and 75% of melanoma patients responded to immunotherapy after FMT (significantly higher than immunotherapy alone) in multicenter Phase II trials using LND101 capsules.