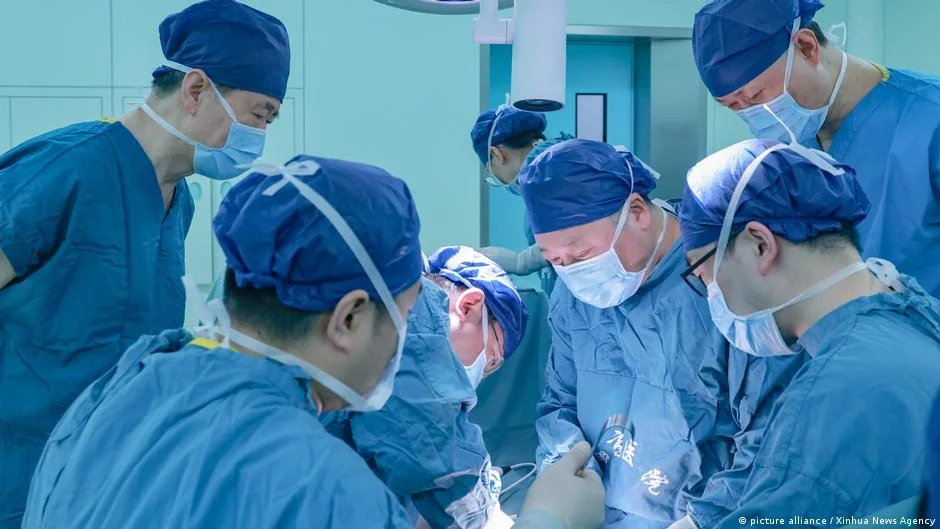
Breakthrough in Organ Transplants: First Successful Pig Liver Transplant in a Living Patient
A 71-year-old man in China received the first successful genetically modified pig liver transplant, marking a significant milestone in xenotransplantation. Although the patient passed away after 171 days due to complications, the procedure demonstrated the potential of animal organs in human medicine, highlighting both progress and ongoing challenges in the field.