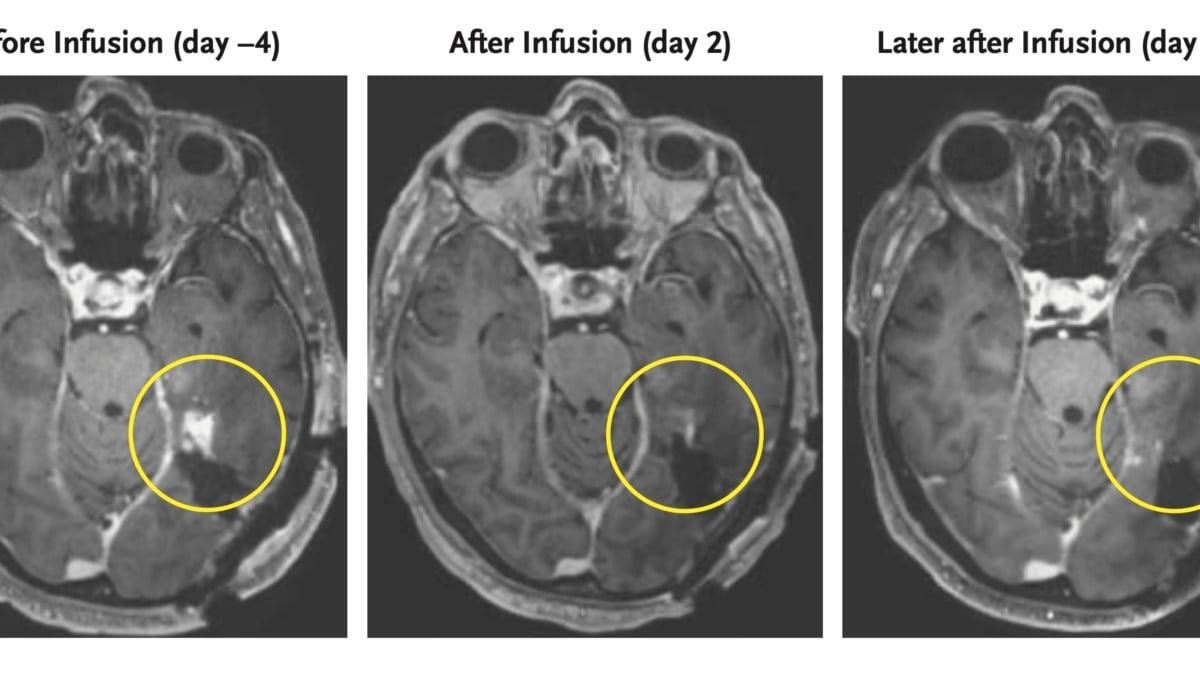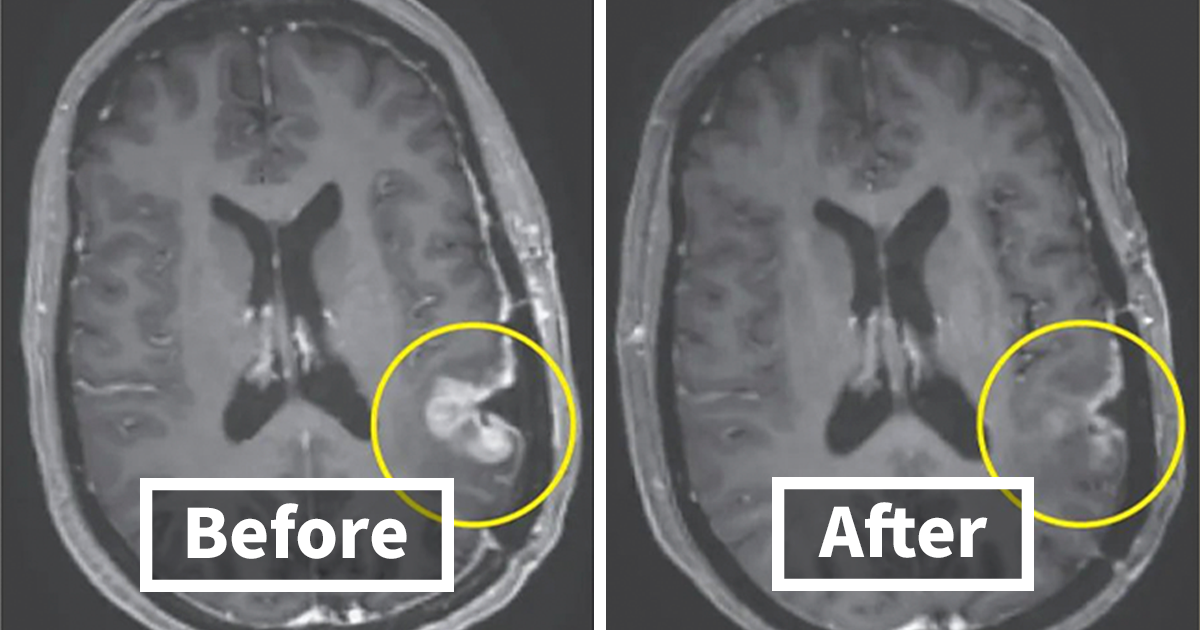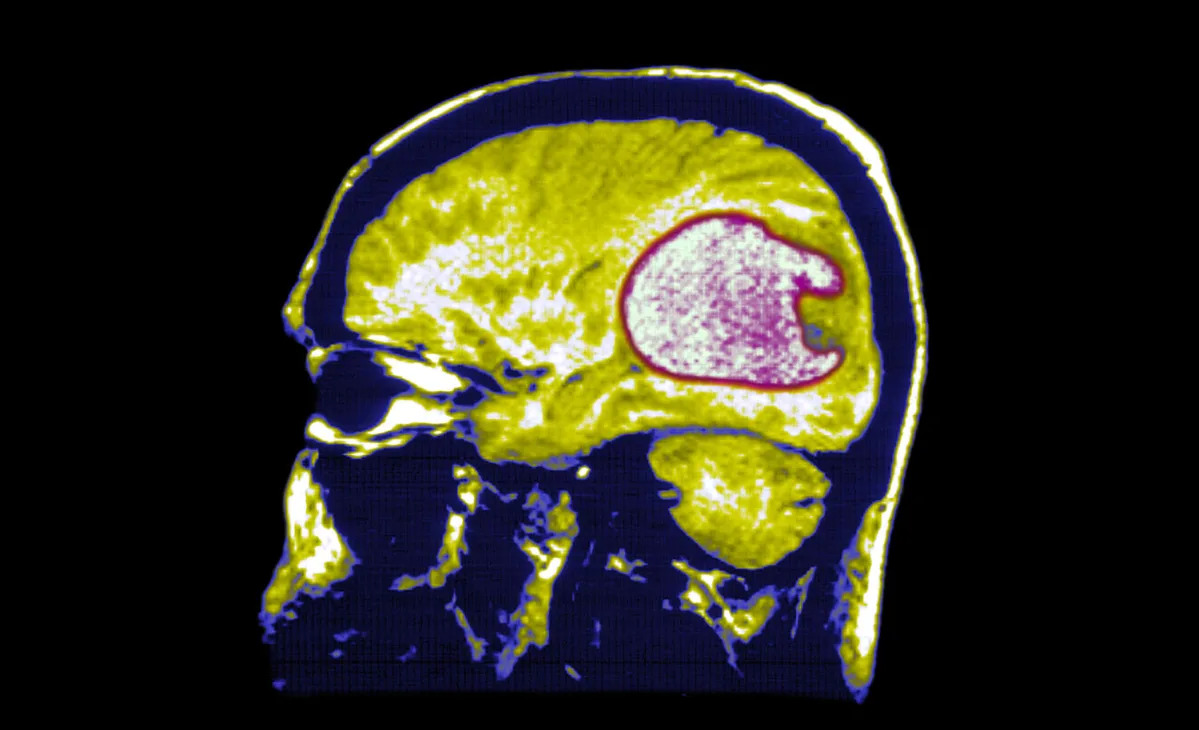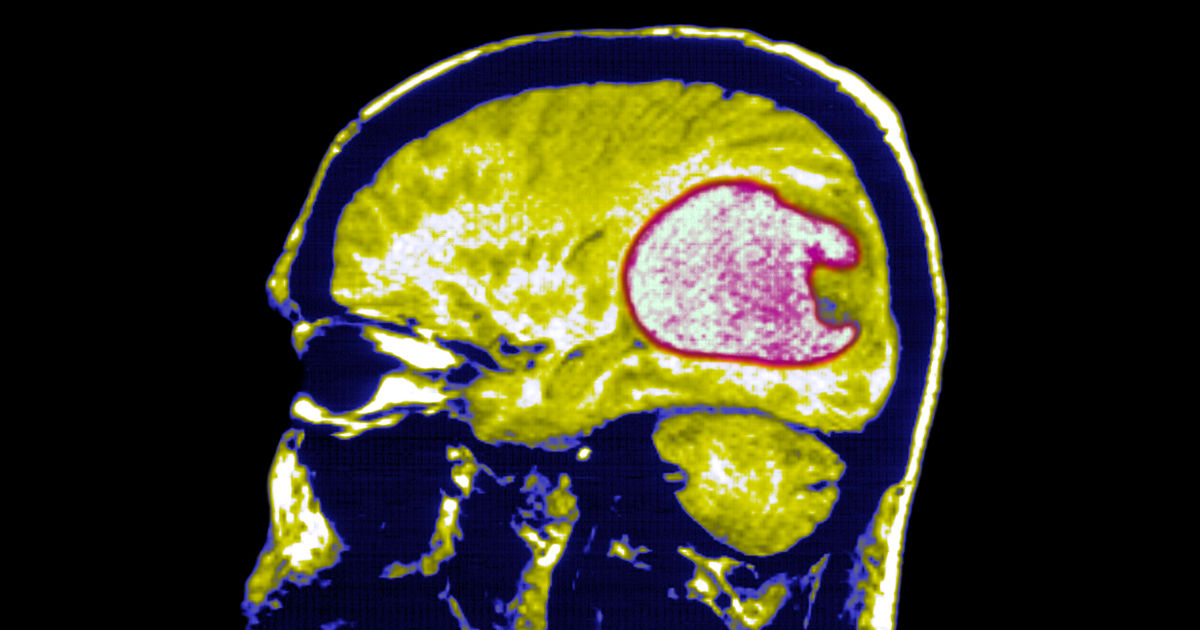
Selective CAR-T targets IGHV4-34, sparing healthy immunity in lymphoma mice
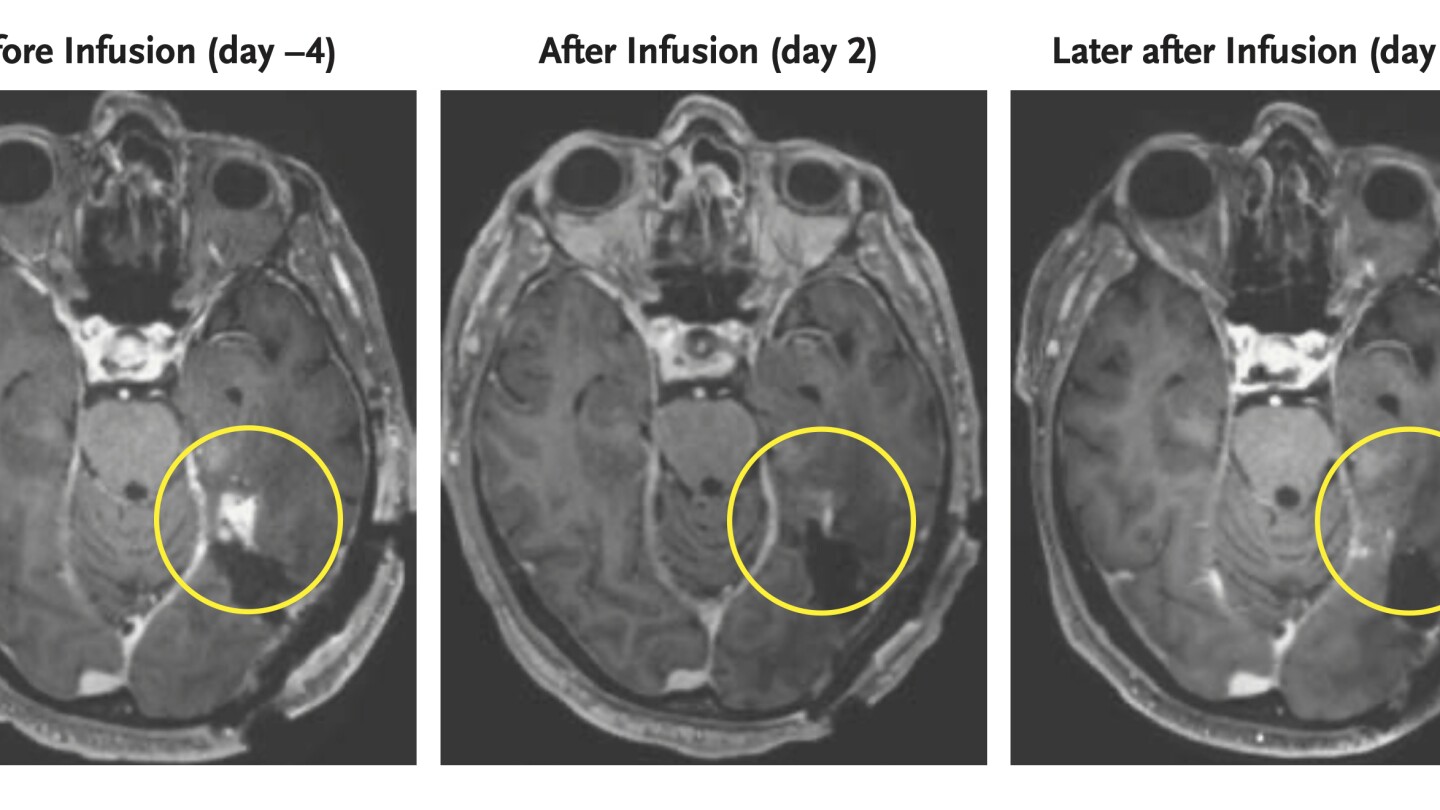
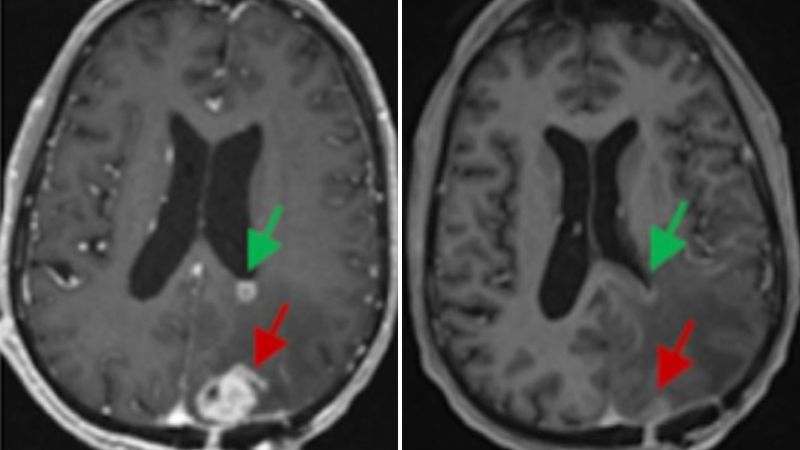
A CART4-34 CAR-T therapy targets the IGHV4-34 gene in B-cell cancer, destroying lymphoma cells in mice as effectively as CD19 CAR-T while sparing healthy B cells and avoiding immune suppression; the approach could also be used to treat autoimmune conditions such as lupus, but human trials are needed.