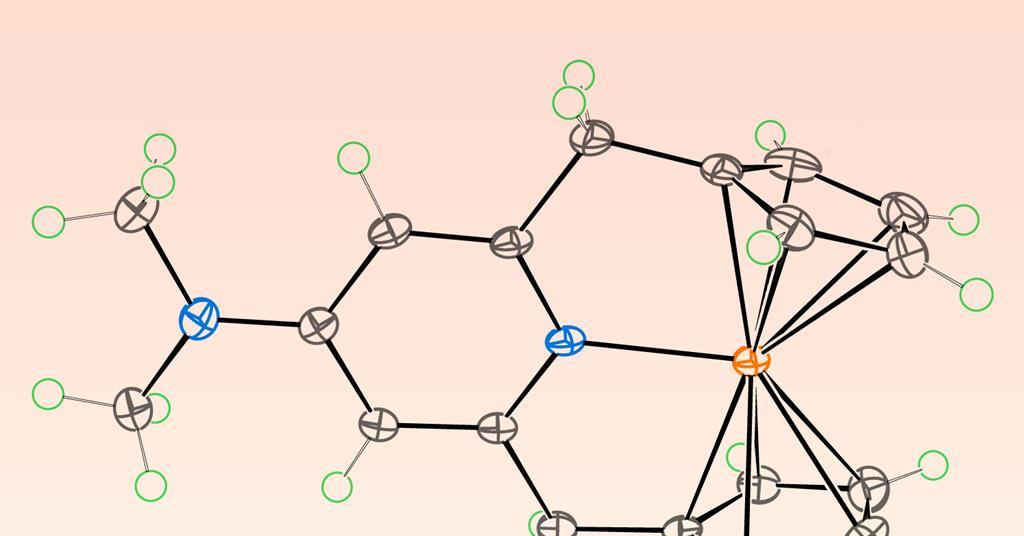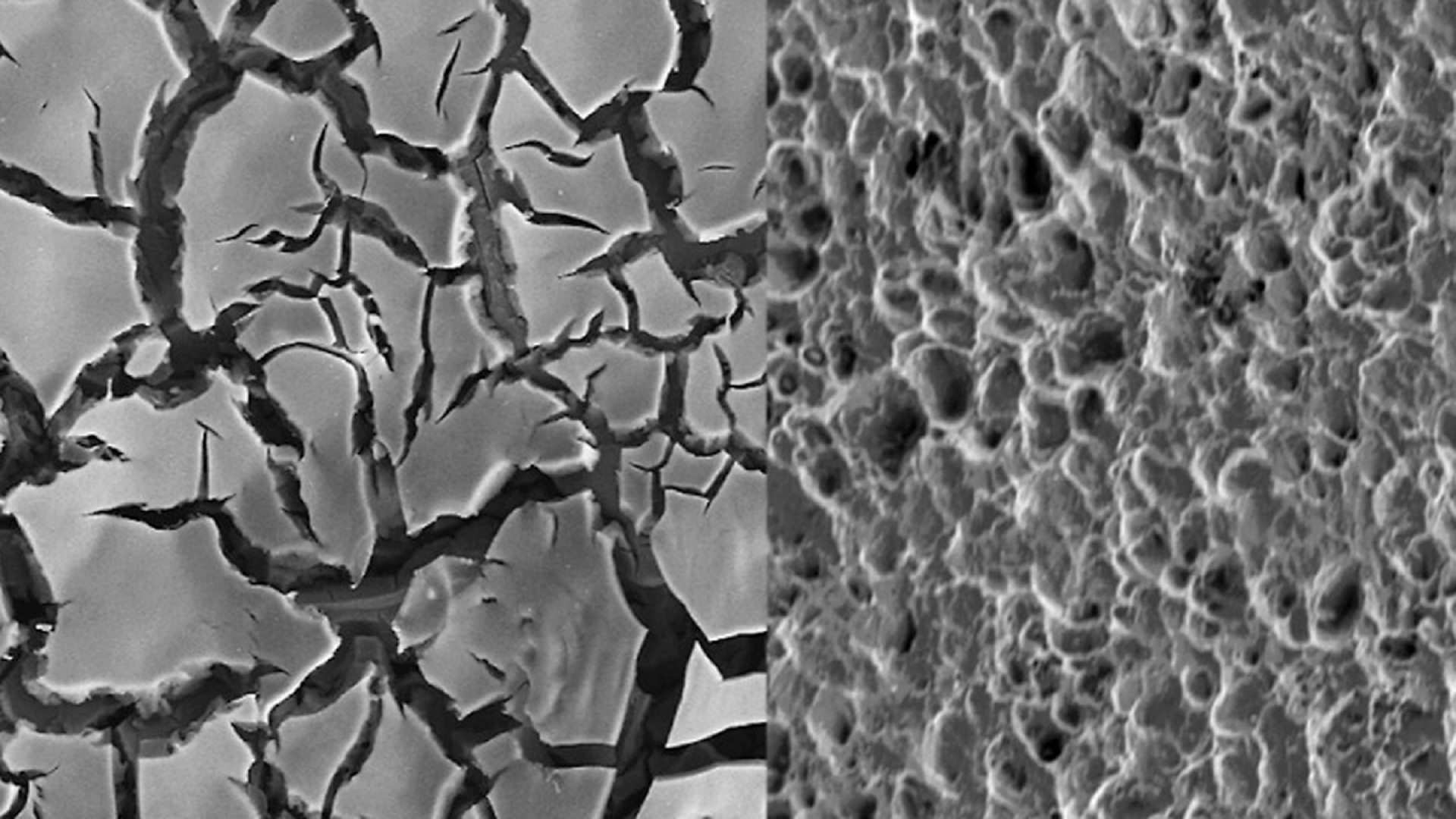
US Scientists Develop Chlorine-Free Water Disinfection Using Quantum Chemistry
US scientists have used quantum chemistry to understand and improve ozone-generating catalysts for chlorine-free water disinfection, aiming to create safer, more sustainable water treatment systems that avoid the drawbacks of chlorine and its byproducts.