Statin Muscle Pain Linked to Calcium Leak Through RyR1, Study Finds
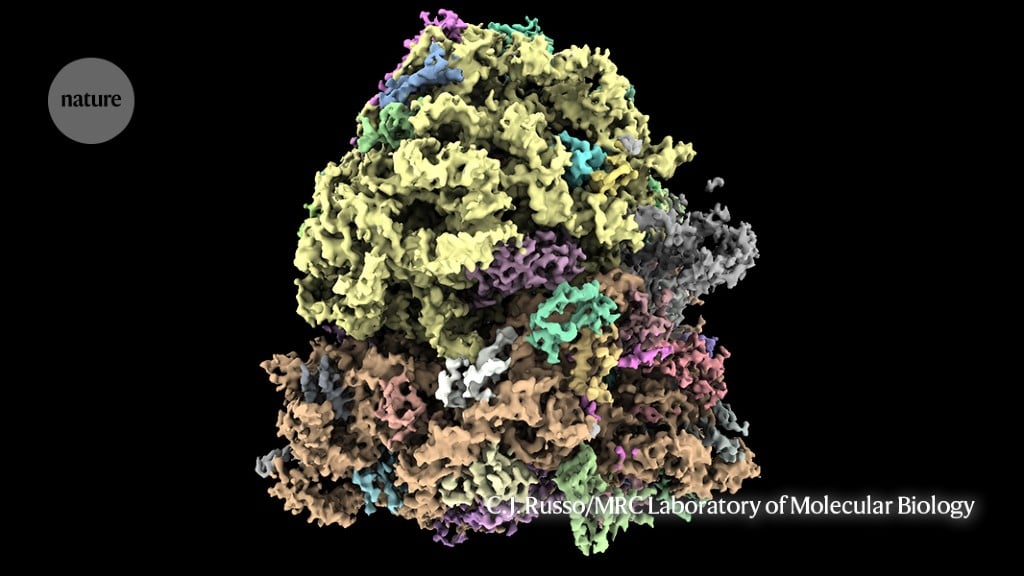
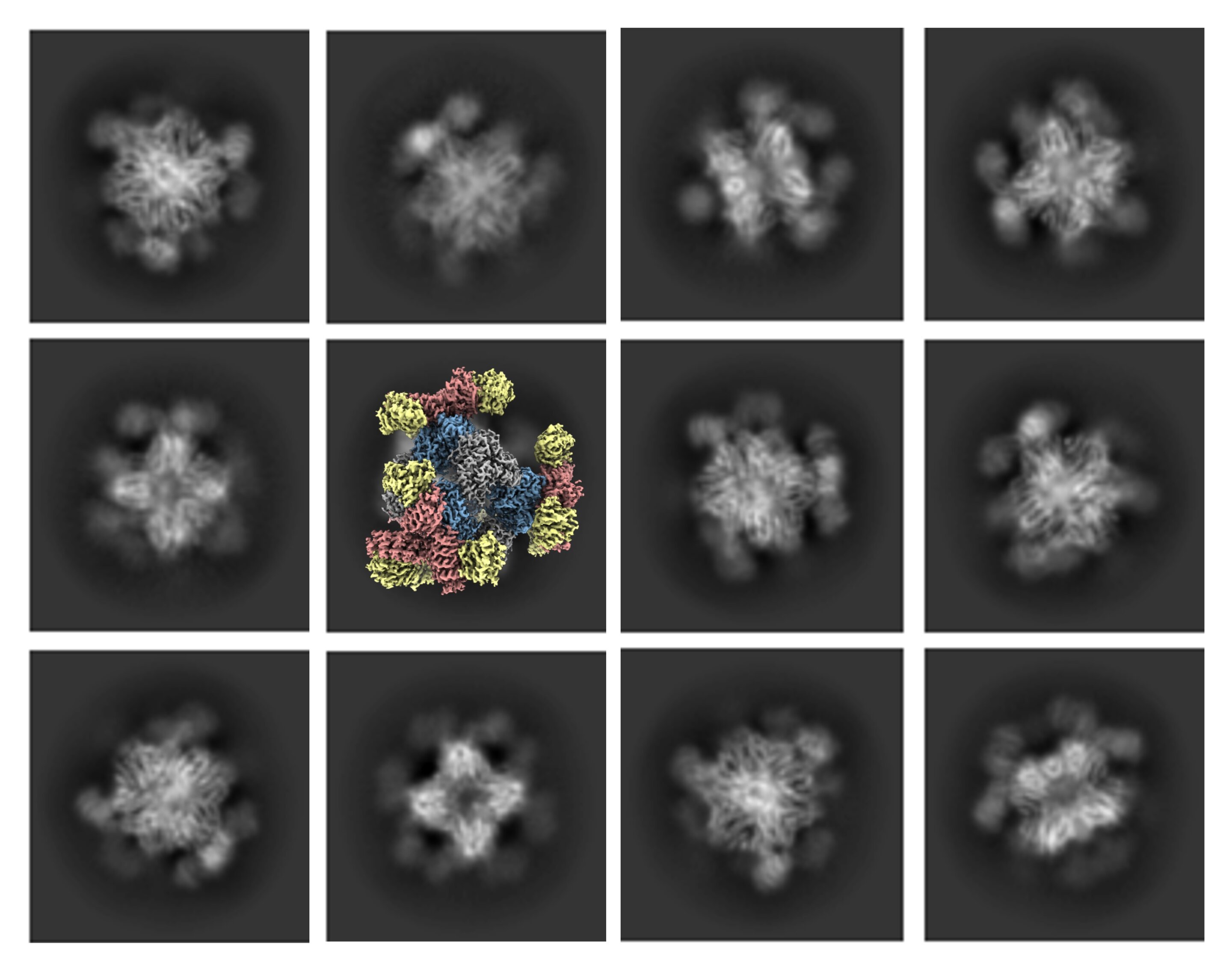
Researchers show that statins bind to the RyR1 muscle channel and force it open, causing a toxic calcium leak that can trigger pain and damage; using cryo-electron microscopy to study atorvastatin, the team mapped a binding pattern and suggested designing safer statins that retain cholesterol-lowering effects without muscle harm.