"Pfizer's RSV Vaccine Shows Promise for High-Risk Adults Under 60"
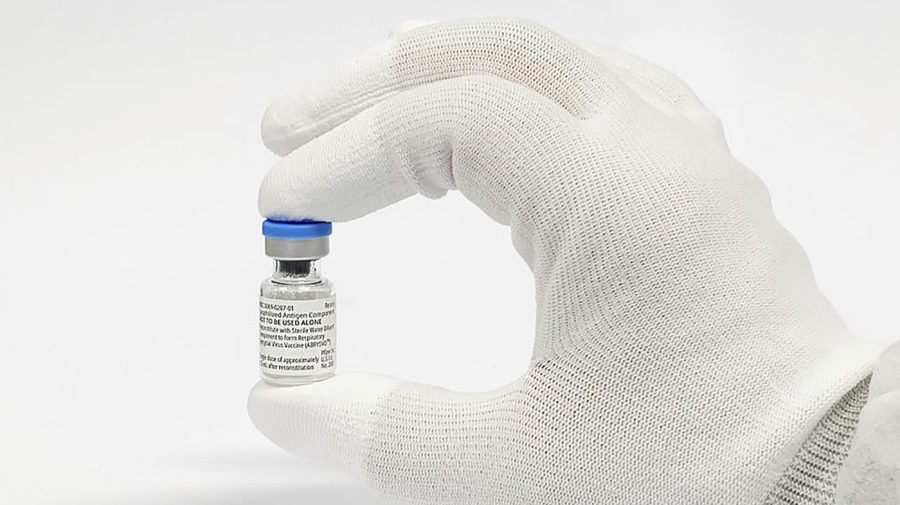
Pfizer's RSV vaccine, Abrysvo, has shown potential to protect high-risk adults aged 18 to 59 from respiratory syncytial virus (RSV) in a late-stage clinical trial, widening its possible use beyond older adults and expectant mothers. The initial data suggests that the vaccine could help protect a wider population from RSV, which causes thousands of hospitalizations and deaths among older Americans and hundreds among infants each year. Pfizer plans to submit the data to regulatory agencies and file for expanded approval of Abrysvo for ages 18 and up, aiming to gain more share of the RSV market. The vaccine elicited an immune response against RSV A and RSV B, and the safety data in high-risk adults ages 18 to 59 was consistent with the results in adults 60 and above.