"Genetic Links Between Diabetes and Cancers Uncovered in Breakthrough Study"
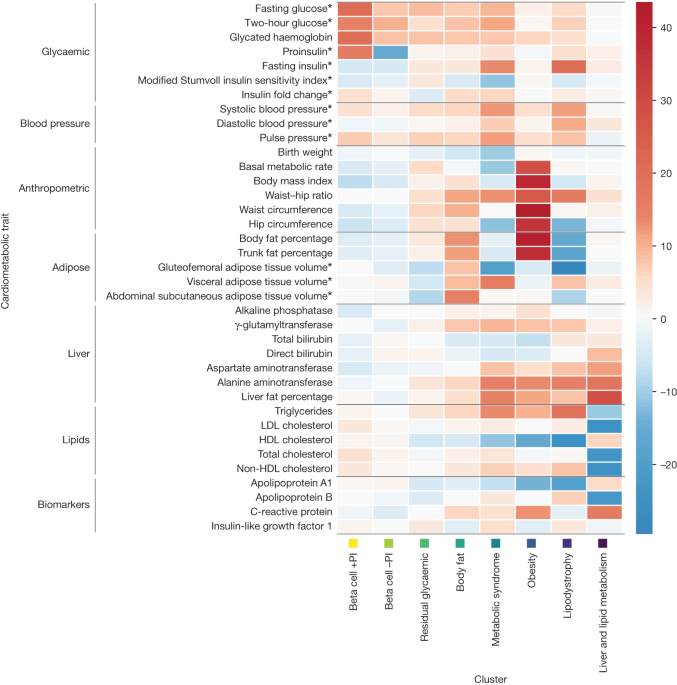
A study by the University of Surrey has identified specific genetic variants that link type 2 diabetes to an increased risk of breast, bowel, and pancreatic cancer. The research, presented at the Diabetes UK Professional Conference, found two key genetic variants that contribute to the development of both type 2 diabetes and certain cancers. The study sheds light on the role of genetically determined factors in the co-occurrence of these conditions, potentially aiding in early identification and personalized prevention and treatment strategies. Experts emphasize the importance of maintaining a healthy diet, weight, and lifestyle to reduce the risks of both type 2 diabetes and cancer.