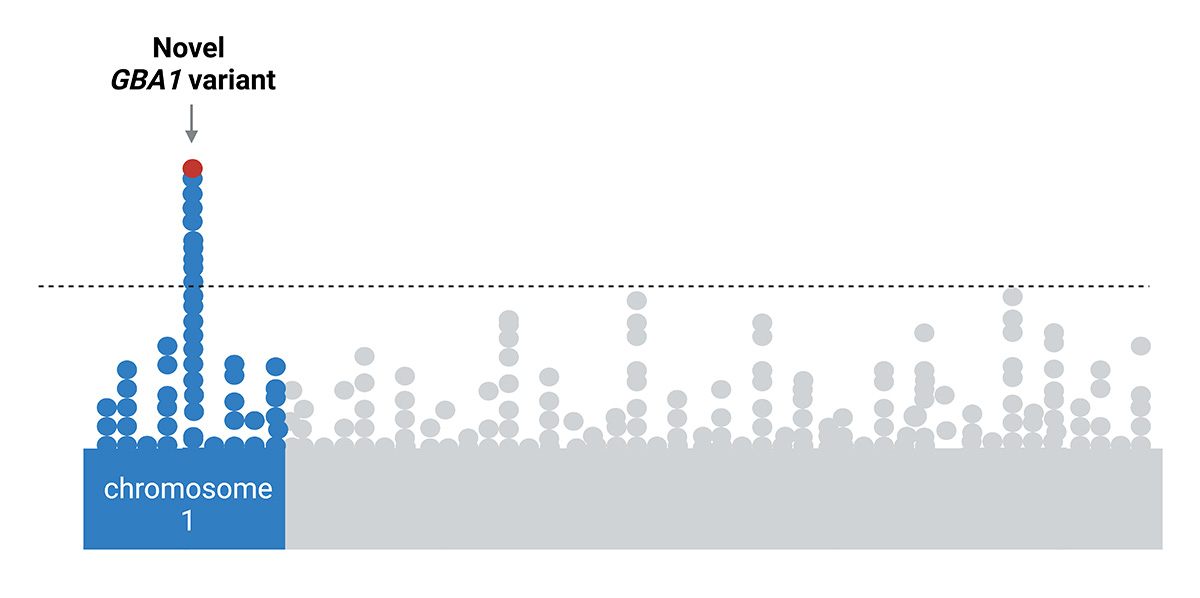
Targeting the APOE Gene Could Revolutionize Alzheimer’s Treatment
Researchers suggest that targeting the Apoe gene, particularly its harmful variants Apoe3 and Apoe4, could potentially prevent most cases of Alzheimer's disease, although challenges remain due to the gene's vital functions and widespread presence in the population.