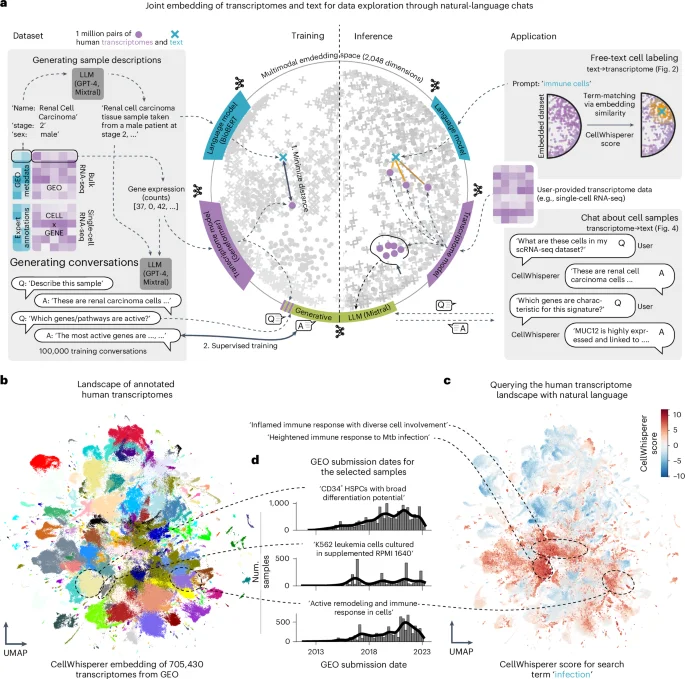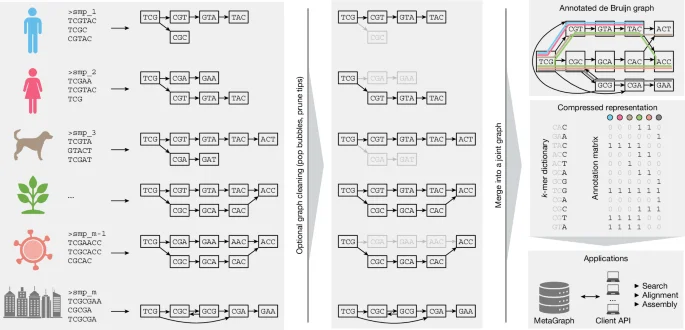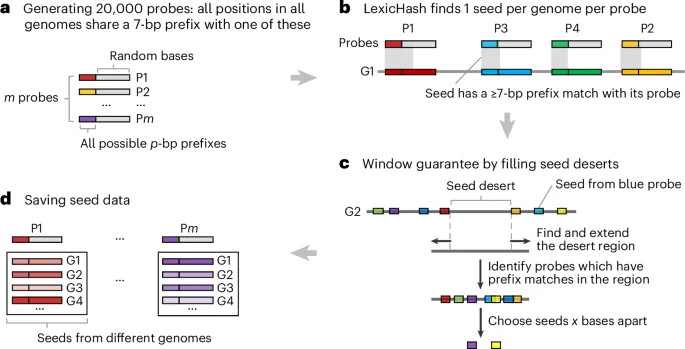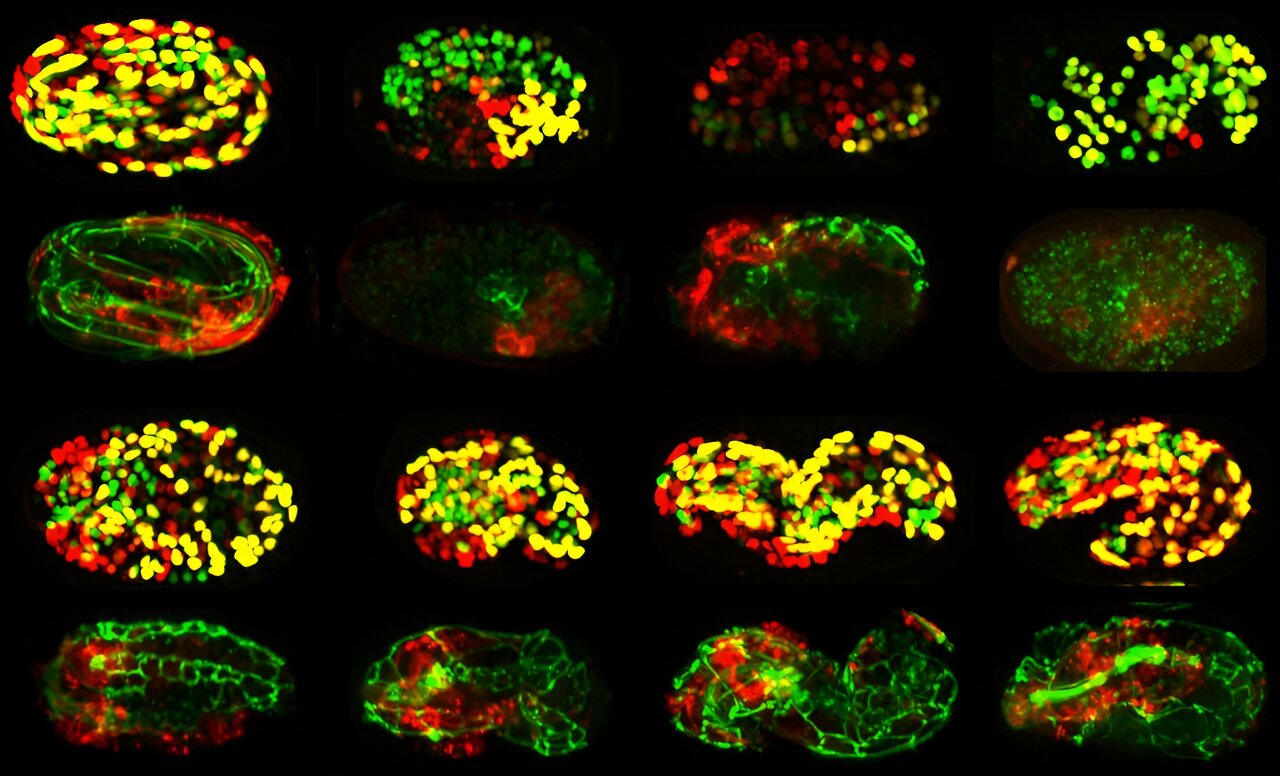
New Tool Unveils Hidden Communication Networks in Cancer
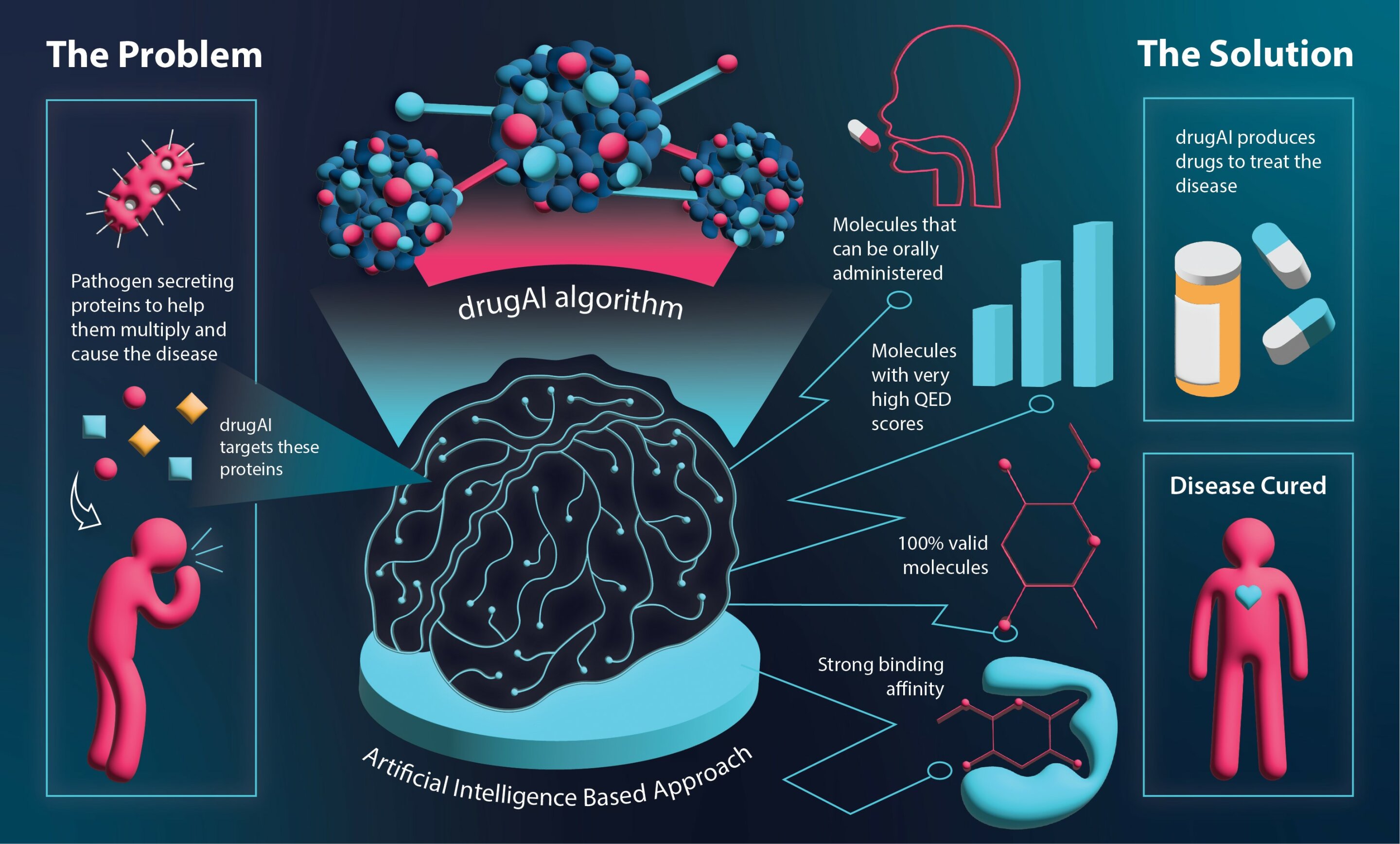
Researchers at the University of Navarra have developed RNACOREX, an open-source software that maps gene regulation networks in cancer, helping to understand tumor behavior and predict patient survival with clear, interpretable results, advancing personalized cancer treatment.