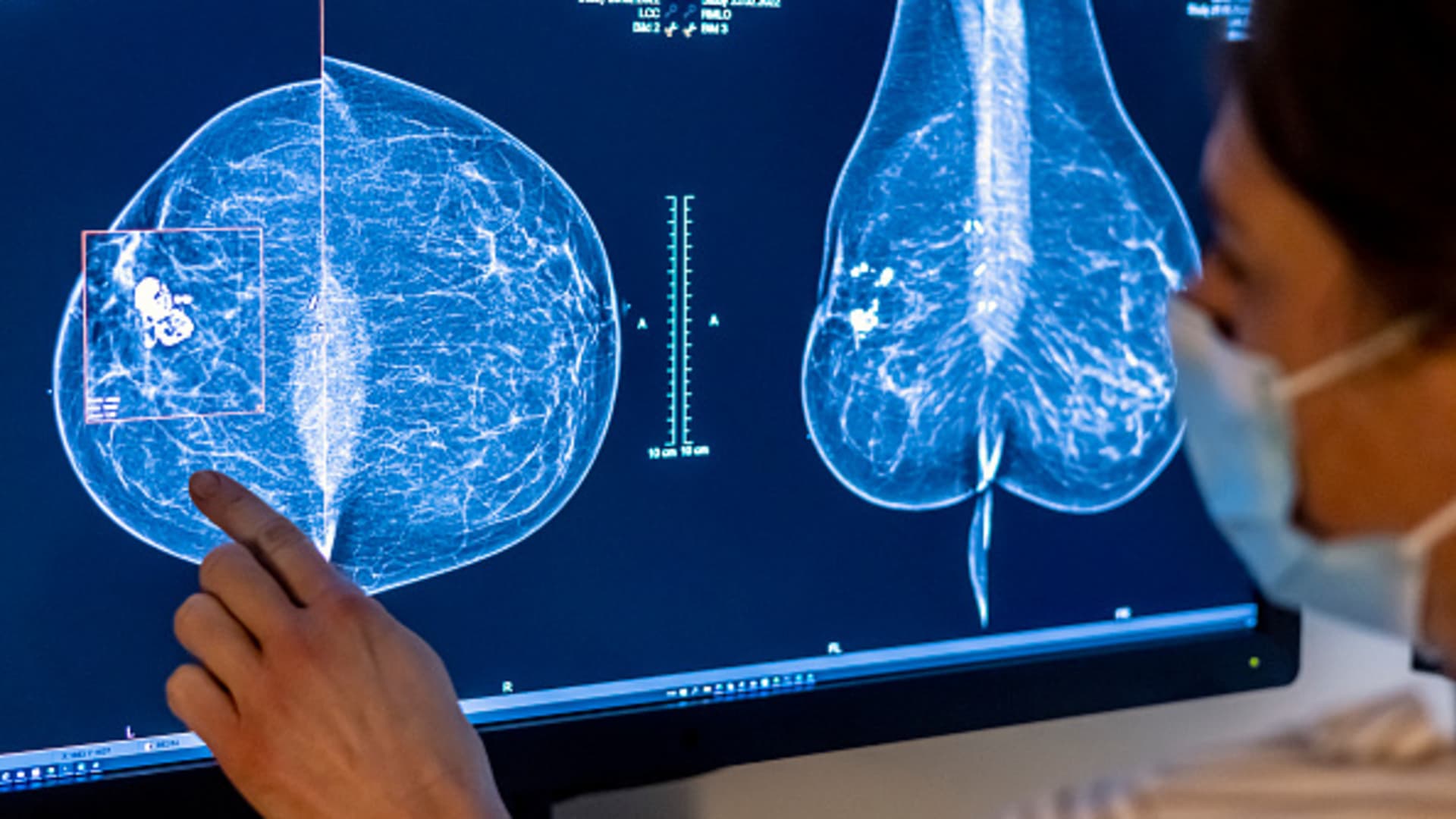
Biotech and pharmaceutical companies are increasingly focusing on antibody-drug conjugates (ADCs) as a promising class of cancer drugs to drive growth, with Johnson & Johnson, Pfizer, and Merck making significant investments in ADCs. The recent rise of ADCs is attributed to advancements in technology, increased confidence in their potential, and the potential for longer market exclusivity. The market for ADCs is expected to continue growing, with estimates suggesting they could account for a significant portion of the worldwide cancer market by 2028. Companies are betting on ADCs to fuel growth and establish themselves as leaders in cancer treatment, with Pfizer's acquisition of Seagen and Merck's licensing agreement with Daiichi Sankyo being notable examples of this trend.