"FDA Approves Madrigal Pharmaceuticals' Rezdiffra as First Treatment for Common NASH Liver Disease"
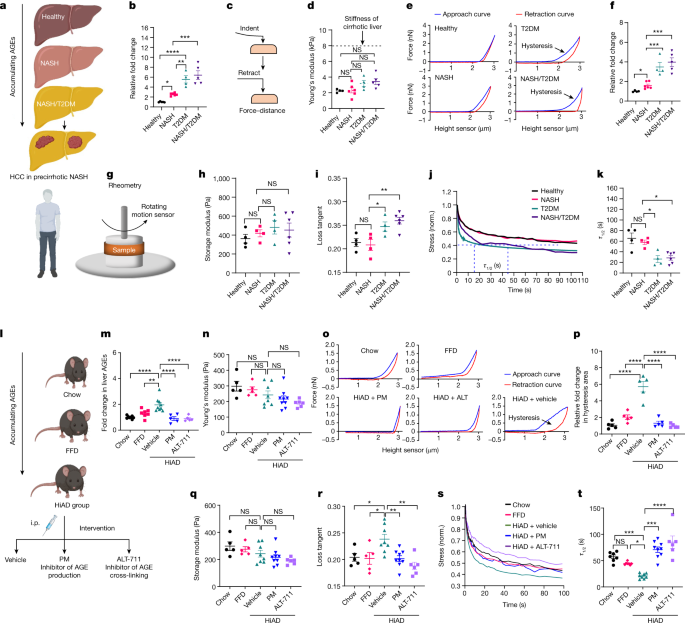
The FDA has approved the first medication, Rezdiffra, for nonalcoholic steatohepatitis (NASH), a common form of liver inflammation associated with obesity and metabolic conditions. The medication, developed by Madrigal Pharmaceuticals, showed promising results in reducing fat accumulation in the liver and achieving NASH resolution with no worsening of fibrosis in clinical trials. It is intended to be used alongside a healthy diet and exercise and is expected to be available next month. The approval marks a significant milestone in NASH treatment and offers hope for patients and healthcare providers.