Contaminated baby formula linked to illness in 36 UK infants
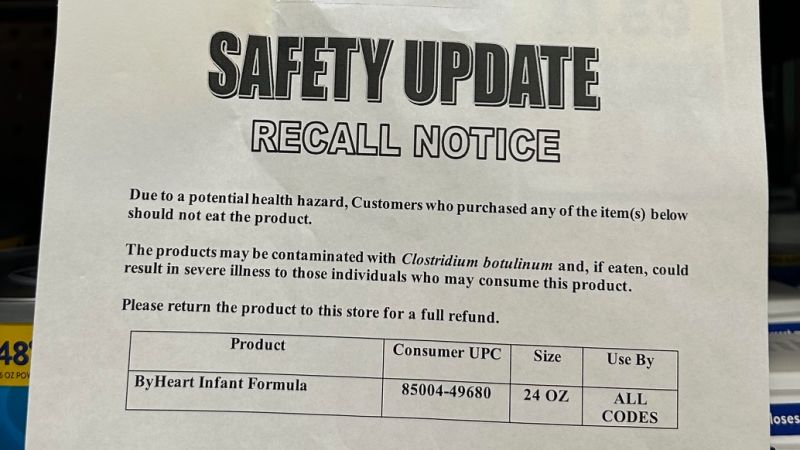
Thirty-six UK infants, mostly under one, have developed vomiting and diarrhoea after consuming formula contaminated with the toxin cereulide. The recalls cover Nestlé and Danone batches, including a Danone 800g batch EXP 31-10-2026 and various SMA products. UKHSA is monitoring the situation and says there is no evidence of widespread illness. The FSA notes the toxin was present in an arachidonic acid oil used in some formulas and urges parents to stop using affected products and switch to an alternative formula while investigators trace the supply chain for further action if needed.