Asteroid 33 Polyhymnia May Harbor Unknown Elements
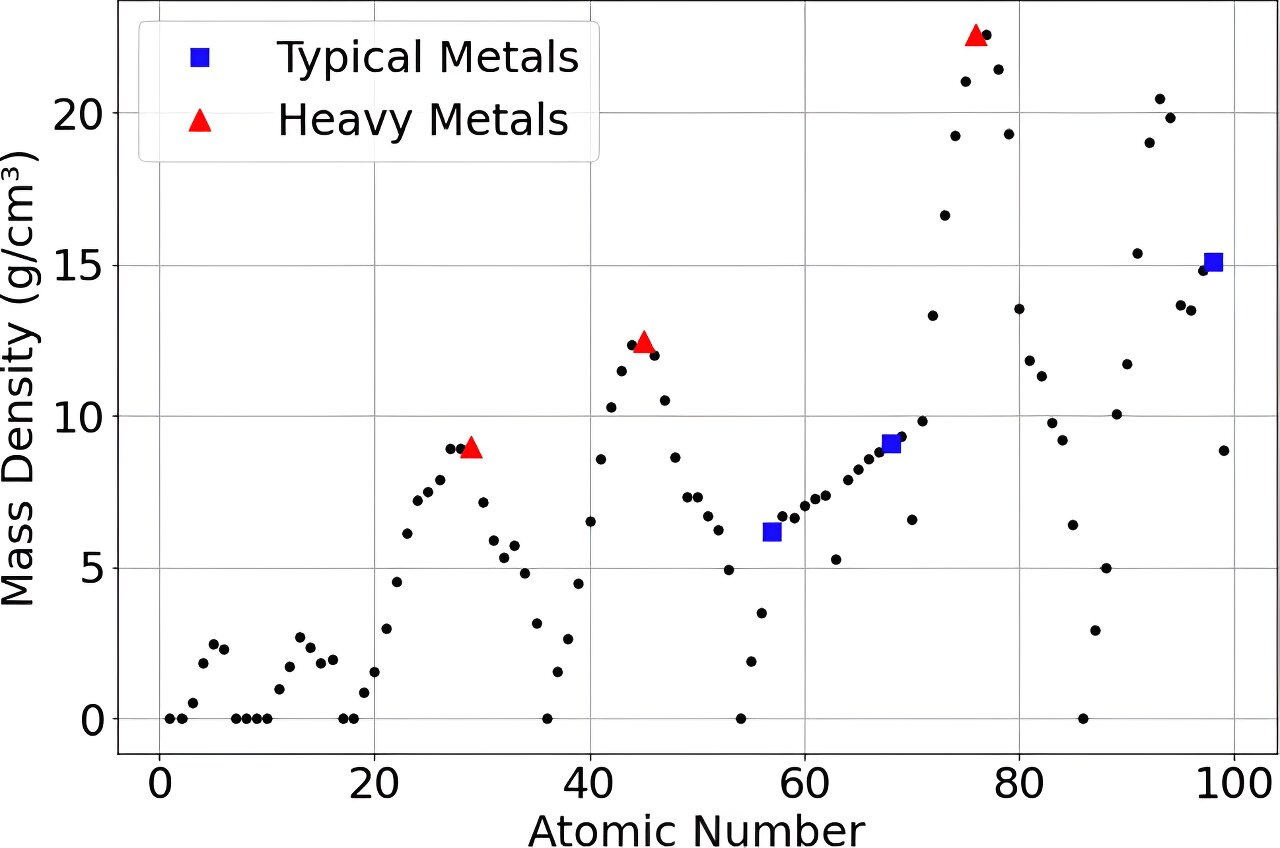
A study suggests that asteroid 33 Polyhymnia may contain ultra-dense, possibly unknown heavy elements beyond the current periodic table, potentially classifying it as a Compact Ultradense Object (CUDO) with a composition that includes stable superheavy elements, which could have implications for space mining and our understanding of matter in the universe.