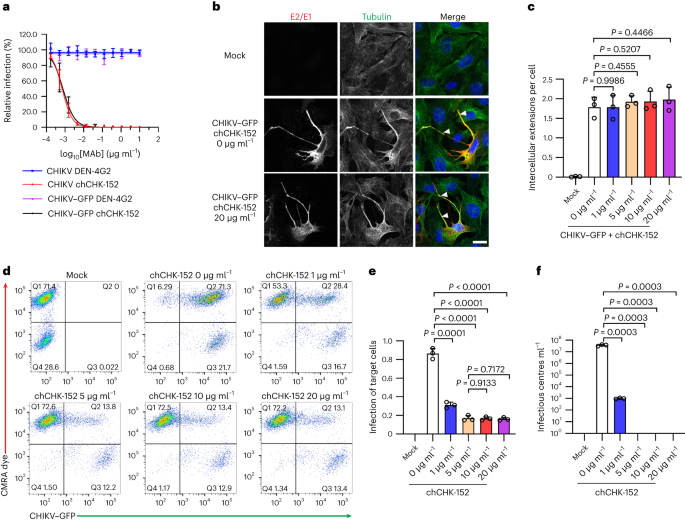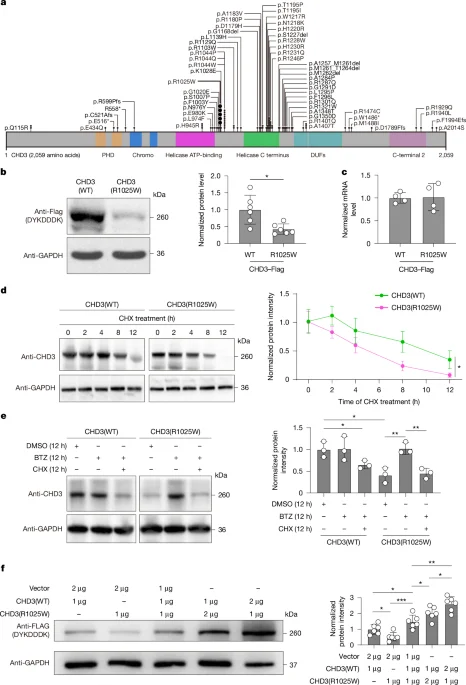
Base editing reverses CHD3-linked neurodevelopmental defects in mice
Researchers created a humanized CHD3 R1025W mouse model of SNIBCPS and used a TadA-embedded adenine base editor delivered by dual AAVs to correct the pathogenic A•T base pair in the brain, restoring CHD3 protein levels and rescuing social, cognitive, and motor deficits; supplementary nonhuman primate work showed widespread neuronal transduction, supporting translational potential for CHD3-related neurodevelopmental disorders.