"Ozempic: Risks, Hollywood Epidemic, and Oprah's Revelation"
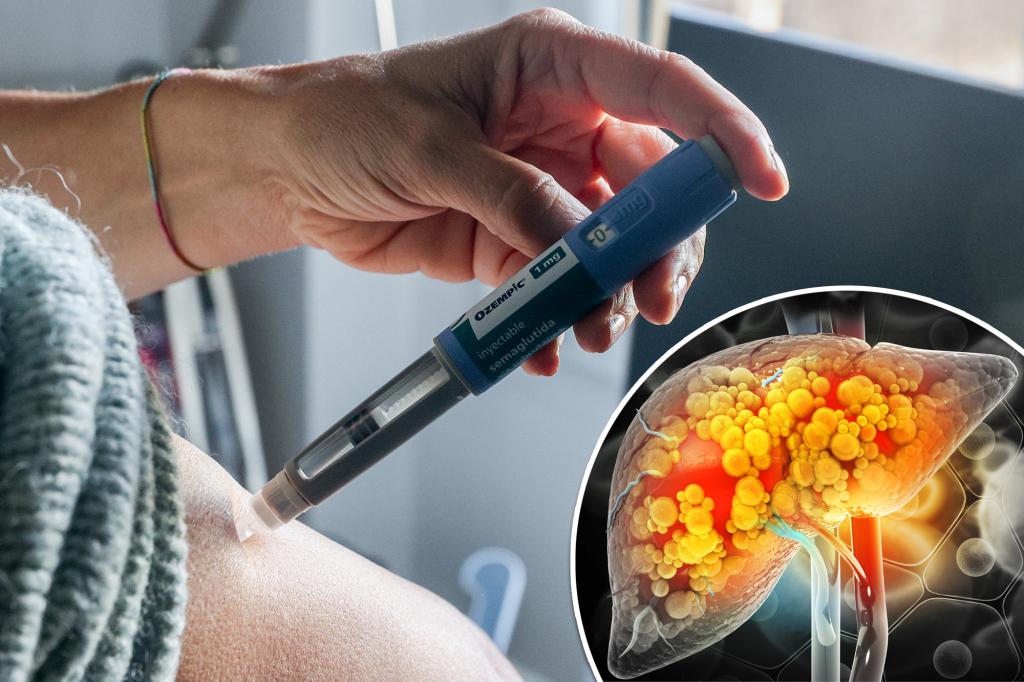
Dr. Mark Hyman warns that the diabetes drug Ozempic can increase certain health risks by up to 900%, citing concerns about its impact on weight regain, muscle loss, bowel obstruction, and pancreatitis. He attributes these side effects to the drug's effects on the gut and urges a focus on addressing the obesity problem instead. In response, Novo Nordisk emphasizes patient safety and collaboration with the FDA.