European Committee Reverses Stance, Approves Alzheimer's Drug
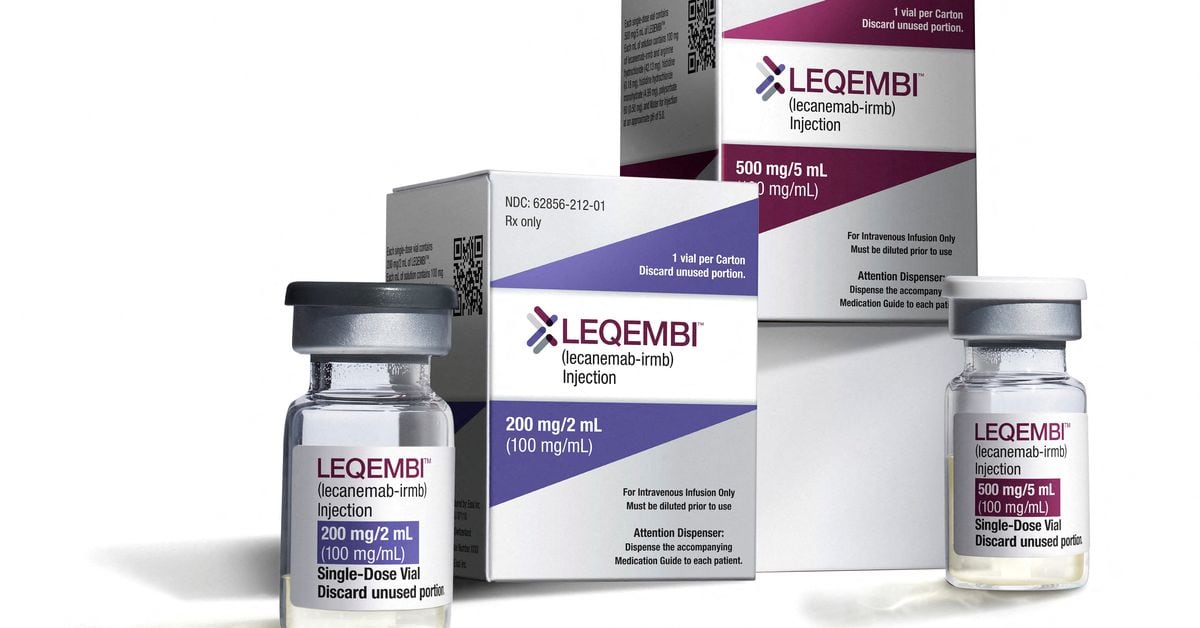
The European Medicines Agency's Committee for Medicinal Products for Human Use has reversed its earlier decision and now recommends approval of the Alzheimer's drug lecanemab, known as Leqembi in the U.S., for early-stage treatment. This comes after initial concerns about side effects like brain swelling and bleeding. Developed by Eisai and co-marketed with Biogen, the drug has shown to slow cognitive decline in Alzheimer's patients. The European Commission is expected to make a final decision on marketing authorization soon.