Eli Lilly's Zepbound and Mounjaro Shortage Expected to Continue Through Q2
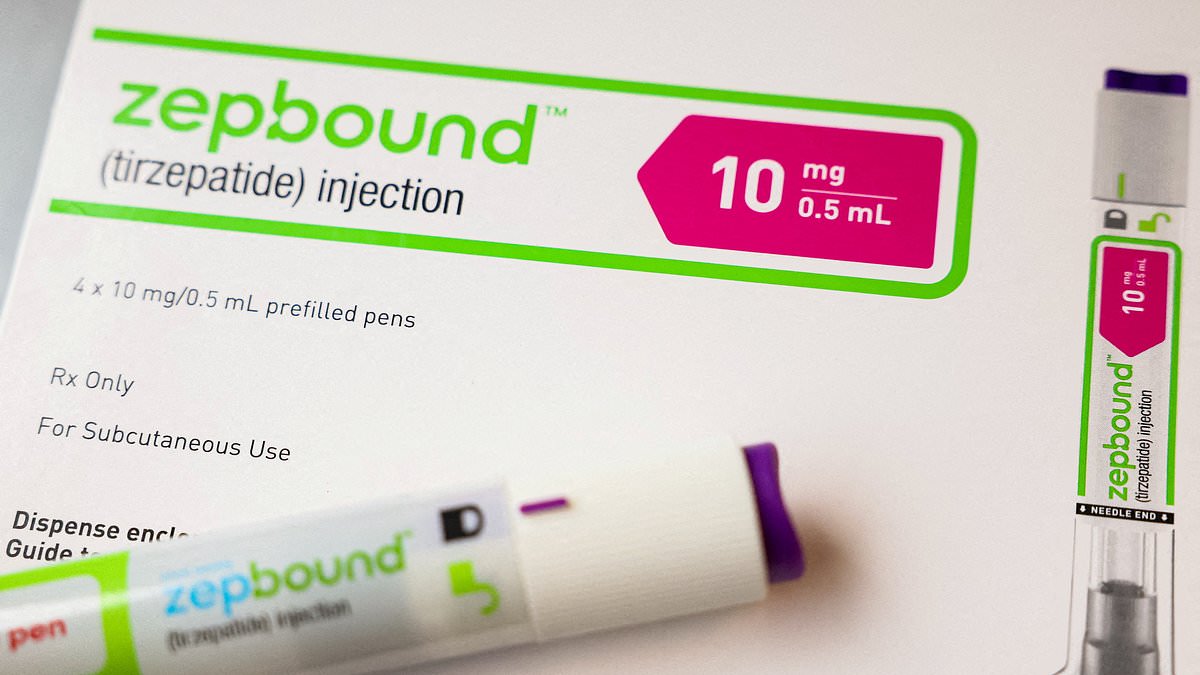
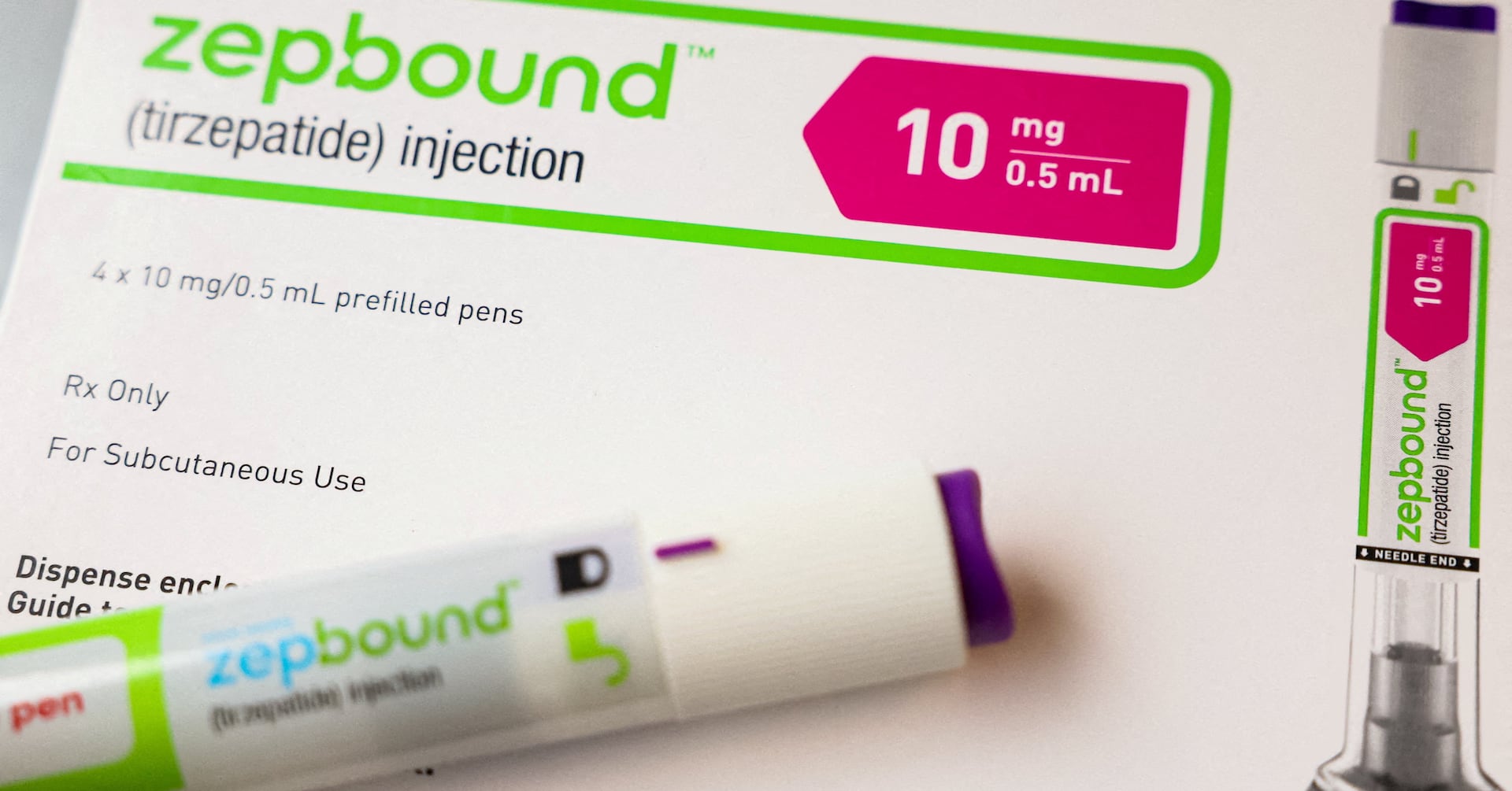
Eli Lilly's weight loss drug Zepbound is facing widespread shortages just months after its approval, with no immediate end in sight. The shortage is attributed to high demand, and the company is working to increase production capacity. Patients are struggling to find the drug, leading to potential disruptions in their weight loss treatment. Some have had to switch to alternative medications due to the shortage.