
Health Safety News
The latest health safety stories, summarized by AI
Featured Health Safety Stories

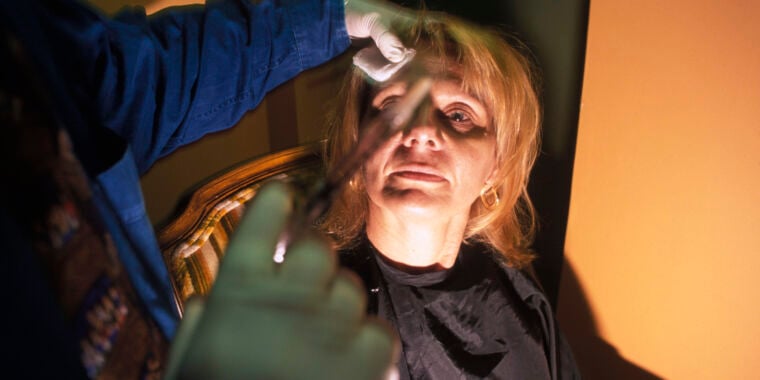
"Counterfeit Botox Injections Trigger Multistate Botulism Outbreak"
Sketchy cosmetic injections of potentially counterfeit Botox have caused a multistate outbreak of botulism-like illnesses, with at least six people falling ill in Tennessee and Illinois. Four of the six required hospitalization. The Centers for Disease Control and Prevention is planning a nationwide alert to notify clinicians of the potentially counterfeit Botox and advise them to be on the lookout for botulism-like illnesses. Health officials urge caution and recommend seeking cosmetic services only from licensed professionals using FDA-approved products to avoid serious health risks.

More Top Stories
"Fatal Health Supplement Linked to Deaths and Hospitalizations in Japan"
CBS News•1 year ago
"Japanese Health Supplement Recall: 5 Deaths and 100+ Hospitalizations"
The Associated Press•1 year ago
More Health Safety Stories

"Health Scare: Japan Recalls Supplements After Deaths and Hospitalizations"
A nationwide recall of a dietary supplement containing red yeast rice has been issued in Japan after two deaths and over 100 hospitalizations raised concerns about potential health risks. The recalled product, "beni koji choleste help," is marketed for lowering cholesterol. The manufacturer, Kobayashi Pharmaceutical, has apologized and is conducting investigations, while health authorities are conducting emergency checks on similar products. The company has suspended online sales in China and removed the products from circulation in Taiwan, and the health ministry is cooperating to investigate the cause and prevent further health issues.

"FDA Issues Warning on Topical Pain Relief Products for Health Risks"
The FDA has issued warning letters to six companies for marketing unapproved and misbranded topical pain relief products containing high concentrations of lidocaine, which pose serious health risks when used before or during certain cosmetic procedures. Consumers are advised not to use over-the-counter pain relief products with more than 4% lidocaine, apply them heavily over large areas of skin, or wrap treated skin with plastic wrap. The FDA urges consumers and healthcare professionals to report any adverse events related to these products and has placed some companies on import alert to prevent their products from reaching consumers.

"CDC Report: Assessing the Safety of Tap Water for Drinking"
A recent CDC report highlights 214 intestine-related disease outbreaks associated with drinking water in the U.S. between 2015 and 2020, resulting in at least 2,140 cases of illness, 563 hospitalizations, and 88 deaths. Biofilms, particularly Legionella bacteria, were identified as common contributing factors. The report underscores the need for better water surveillance, prevention, and outbreak response programs. To ensure safer drinking water, the CDC recommends boiling water, using certified water filters, purchasing distilled or sterile water for specific uses, and regularly cleaning and maintaining water-related appliances.
"Unveiling the DNA Damage: The Alarming Truth About Vaping and Its Cancer Risk"
Vaping, once considered a safer alternative to smoking, is now under scrutiny as studies reveal potential health risks, including cell changes linked to cancer, toxic metal exposure, respiratory inflammation, and cardiovascular effects. Concerns are heightened by the increasing popularity of vaping among teens and children, leading to hospitalizations and prompting legislation to restrict availability and curb underage usage. Despite being touted as a smoking cessation aid, the addictive nature of nicotine in vapes has led to a booming industry with potential long-term health consequences yet to be fully understood.

"Understanding the Risks of Overconsumption: The Dangers of Daily Vitamin D Supplements"
After an 89-year-old man died from vitamin D toxicity, a U.K. coroner is urging the country’s Food Standards Agency to improve supplement labeling. The man had been taking vitamin D supplements for nine months before his death, and the coroner's report alleges inadequate warnings about supplement overdose on the packaging. Experts recommend daily vitamin D intakes of 600 IU for adults ages 19 to 70, and 800 IU for those 71 and older, with a maximum safe daily intake of 4,000 IU. Vitamin D toxicity can lead to hypercalcemia, causing symptoms such as weakness, fatigue, and bone pain. It's important to monitor vitamin D intake and consult a doctor before taking supplements, as excessive levels can be dangerous.

Tragedy Strikes: 9 Dead After Consuming Sea Turtle Meat in Zanzibar
Eight children and one adult died on Pemba Island, Africa, after consuming sea turtle meat, a local delicacy known for its food poisoning dangers. Dozens more were hospitalized, with lab tests confirming the link between their illness and the consumption of sea turtle meat. This incident echoes a similar one in November 2021. Sea turtle meat can cause chelonitoxism, a deadly form of food poisoning, particularly affecting children and nursing babies. Authorities have urged people not to eat sea turtle meat in the wake of these tragic poisonings.

"FDA Recalls Cinnamon Brands Over Lead Contamination"
The FDA has issued a warning about ground cinnamon sold at discount retailers, including Dollar Tree and Family Dollar, due to high levels of lead contamination, which could be unsafe for people, especially children, with prolonged exposure. The agency urged suppliers to voluntarily recall the products, and Dollar Tree and Family Dollar have already removed the affected cinnamon from their shelves. Consumers are advised to discard any of the mentioned cinnamon products they have at home. The FDA's targeted survey was prompted by a previous recall of lead-tainted cinnamon applesauce pouches, and while no illnesses have been reported in connection with the contaminated cinnamon, long-term lead exposure can cause serious health issues, particularly in children.

"FDA Alerts Consumers to Lead Contamination in Six Ground Cinnamon Brands"
The FDA has issued a safety alert warning consumers to stop using certain ground cinnamon products sold by retailers including Family Dollar, Dollar Tree, and Save A Lot, due to elevated levels of lead. The tainted products from six different distributors have been identified, and the FDA has recommended a voluntary recall. Consumers are advised to check their pantries for the impacted products and to consult a healthcare provider if they believe they've been exposed to elevated levels of lead. Long-term exposure to lead can lead to adverse health effects, especially in children, and the recall specifically targets the identified brands, not all cinnamon products.

FDA Issues Alert for Lead-Tainted Ground Cinnamon from Six Brands
The FDA has expanded its investigation into lead contamination in cinnamon products, warning consumers to stop using ground cinnamon from six brands due to elevated levels of lead. This comes after an earlier recall of cinnamon-flavored applesauce pouches. The agency has advised manufacturers to recall these products and consumers to stop using them, as prolonged exposure may be unsafe. The concentrations of lead in the ground cinnamon products ranged from 2 to 3.4 parts per million, far lower than the levels found in the recalled applesauce pouches. The FDA is recommending recalls of ground cinnamon from six distributors and urges anyone who may have been exposed to elevated levels of lead to consult a healthcare provider.

"Benzene Contamination in Acne Products Sparks FDA Recall Petition"
Valisure, an independent testing laboratory, has detected high levels of the cancer-causing chemical benzene in various benzoyl peroxide (BPO) acne products from major brands, prompting a petition to the FDA for a recall. The affected products include those from Proactiv, Clinique, Clearasil, and more, with some containing up to 12 times the allowed amount of benzene. Dermatologists are urged to discuss the risks with their patients, as benzoyl peroxide breaks down into benzene, posing an inhalation risk and potential long-term cancer risk. This discovery raises concerns about the safety of over-the-counter and prescription acne treatments and highlights the need for stricter regulations to protect consumer safety.
