
Health Business News
The latest health business stories, summarized by AI
Featured Health Business Stories


"Zoetis Stock Plummets Amid Concerns Over Pet Arthritis Drugs Sickening Dogs and Cats"
Zoetis Inc's stock plummeted after reports suggested that its pet arthritis drugs, Librela and Solensia, may have caused adverse effects in dogs and cats. The FDA approved these drugs for pain associated with osteoarthritis in pets, marking a significant development in pet healthcare. However, some pet owners have linked the drugs to adverse effects and deaths in their pets, leading to scrutiny and thousands of reports of side effects. Zoetis stated that these side effects represent a fraction of the shots given and that neither the company nor researchers have identified a connection between the drugs and the reported adverse effects. Additionally, the European Commission initiated an investigation into whether Zoetis hindered the market launch of a competing pain medication for dogs.

More Top Stories
"Philips Agrees to Overhaul Manufacturing After Sleep Apnea Machine Recall"
Yahoo! Voices•1 year ago
More Health Business Stories

"Lilly's Mounjaro: Britain's New Weight-Loss Drug Launch"
Eli Lilly's weight-loss drug Mounjaro is being launched in the UK, making it the fourth European country to introduce the highly-anticipated obesity drug. The drug, also approved for diabetes, will be available for both private and NHS patients. With a high demand indicated by the popularity of rival weight-loss drug Wegovy, pharmacies are preparing for strong interest. The UK will be the first major market to receive Mounjaro in a more convenient pre-filled injection pen, with a 4-week course priced at around £215 for private patients. The concentration of the active ingredient in the shots needs to build up over months to reach a maintenance level to slowly accustom the body to the appetite-suppressing peptides.
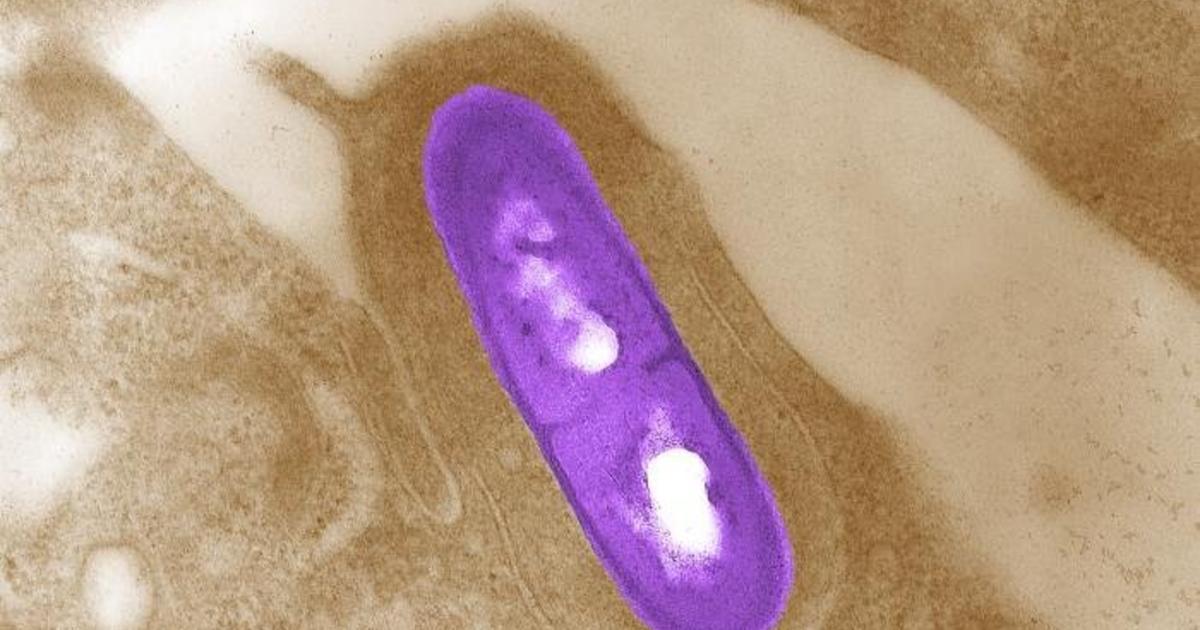
"Listeria Outbreak Triggers Product Recalls at Major Retailers"
A widespread recall of dairy products linked to a deadly listeria outbreak has expanded to include items sold at major retailers such as Costco, Trader Joe's, and Walmart. The recall encompasses dips, dressings, salad and taco kits, with at least 26 people in 11 states affected by the outbreak. The potentially tainted products were distributed by various companies, and consumers are urged to discard them due to the risk of listeria contamination, which can be particularly dangerous for pregnant individuals, newborns, the elderly, and those with weakened immune systems.

"Ethical Concerns Surrounding Ozempic and Other Injectable Weight Loss Drugs"
In the podcast "Chasing Life," CNN's Dr. Sanjay Gupta discusses the booming business of weight loss drugs, such as Ozempic, Wegovy, Mounjaro, and Zepbound, with colleague Meg Tirrell. The demand for these medications has surged, with U.S. healthcare providers writing over 9 million prescriptions in the last three months of 2022 alone, despite their high cost of about $1,000 a month. The rise of these drugs, particularly GLP-1 drugs, has surprised many due to previous failures in the weight loss medication space. The industry is projected to reach $100 billion in annual revenue by 2030, with companies like Novo Nordisk and Eli Lilly dominating the market and struggling to keep up with demand.
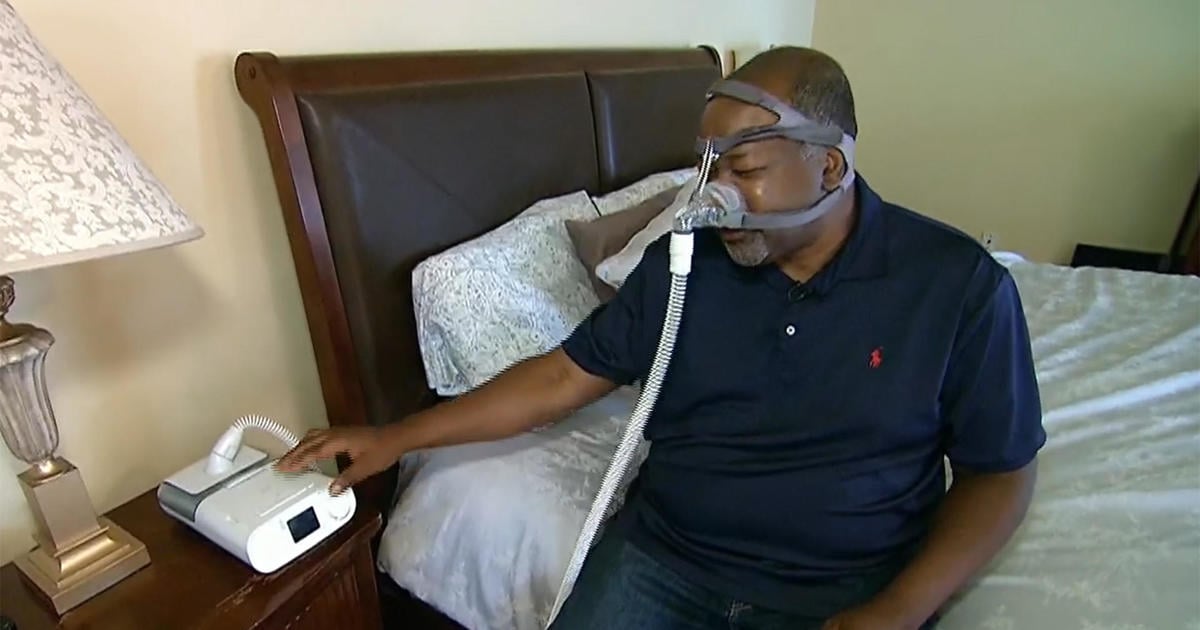
"FDA Links 561 Deaths to Recalled Philips Sleep Apnea Machines"
The FDA has reported 561 deaths linked to recalled Philips devices used to treat sleep apnea and other breathing disorders, with over 116,000 medical device reports of foam breaking down in the machines. Philips has agreed to a $479 million settlement and will stop selling the machines in the U.S., while continuing to service existing ones. Users affected by the recall can file claims for financial losses, and the deadline for submissions is August 9, 2024. The settlement does not affect claims for personal injuries or medical monitoring relief, and Philips denies conclusive data linking the devices to the reported deaths.
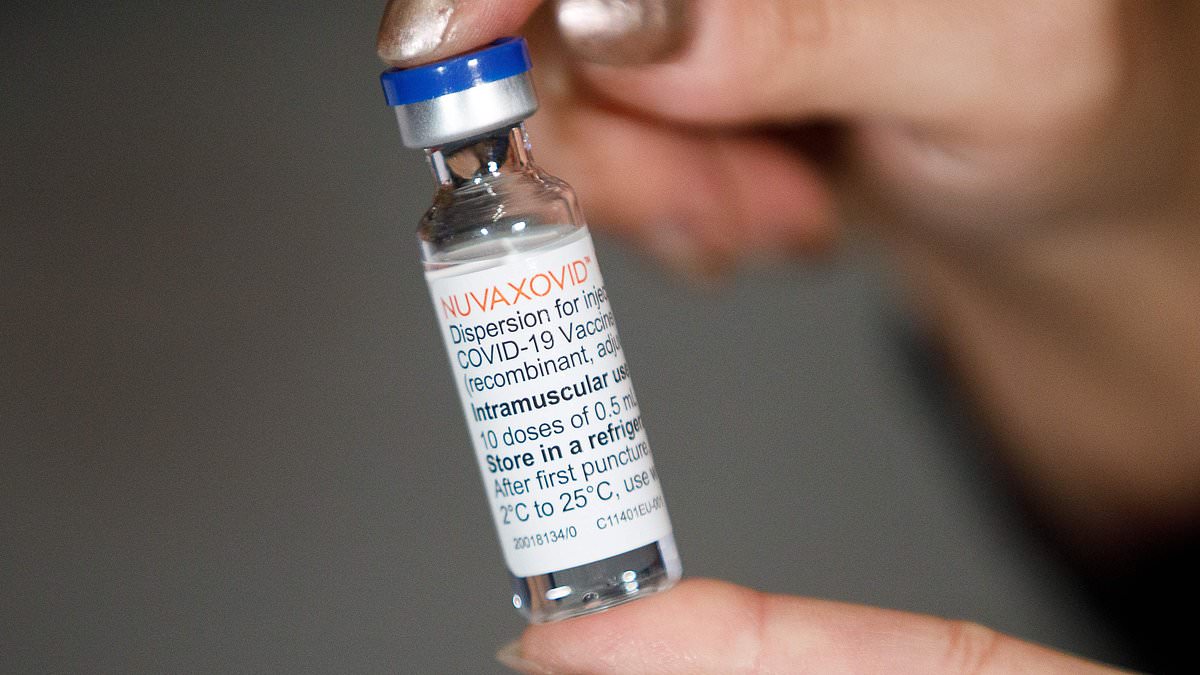
"High Street Pharmacies to Offer Private Covid Vaccines for £45 in the UK"
Chemists in the UK will start offering Covid vaccines privately, with Brits able to buy them for as little as £45 within weeks. The move comes as part of a huge shake-up to the scheme, allowing high street pharmacies to sell the Novavax vaccine privately. This change is expected to increase accessibility and uptake of Covid vaccines, with experts calling for private sales since the threat of the virus began to fade. The move is seen as a way to ease pressures on the NHS and free up GPs to deal with more serious cases, although concerns remain over the spread of the virus and the capacity of pharmacies due to closures and staff shortages.

"Philips Halts US Sales of Sleep Apnea Machines Due to Safety Concerns and Recalls"
Philips is stopping sales of its recalled sleep apnea machines in the US due to foam particles being released into users' airways, leading to potential health risks. The company has reached a consent decree with the Department of Justice and FDA and will not sell new CPAP or BiPAP devices in the US until meeting FDA requirements. Philips has set aside €363 million to address the FDA's requirements and will continue to provide service to sleep and respiratory devices in the US while also selling the machines outside of the US.

"Philips Halts US Sales of Sleep Apnea Machines Amid Ongoing Safety Concerns"
Philips will stop selling its sleep apnea machines in the US after a global recall due to potential health risks associated with the polyester-based polyurethane foam used in the devices. The company has agreed to a consent decree with the FDA and the Department of Justice, potentially costing it nearly $400 million. The recall affects approximately 5 million CPAP machines and ventilators, with concerns about inhaling or swallowing foam particles and chemicals. Philips will continue to service existing machines in the US but cannot sell new ones until required changes are made.
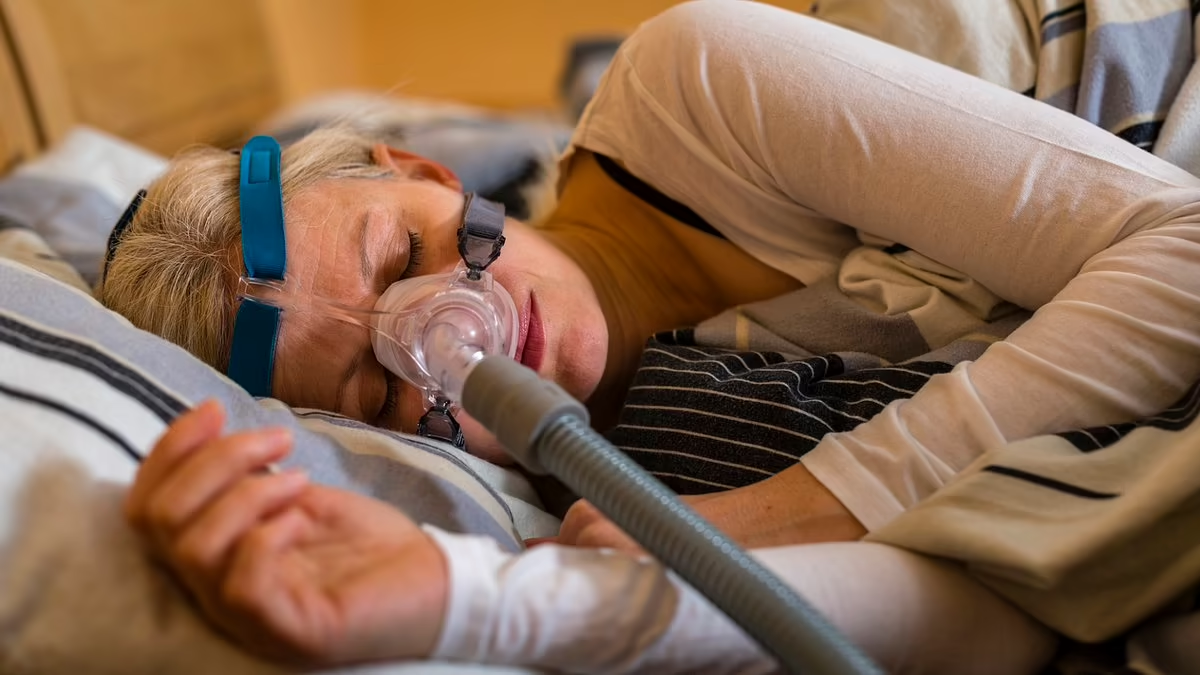
"Philips Suspends Sales of Sleep Apnea Machines Over Cancer Concerns"
Philips is halting sales of its sleep apnea machines due to concerns about potential cancer risks associated with the devices. The company had previously recalled over 5 million CPAP machines in 2021 after discovering that they were blowing gas and foam into users' airways, increasing the risk of tumors. The FDA and Justice Department have forced Philips to stop new sales until the issue is addressed. The company is facing over 700 lawsuits alleging that the devices caused patients to develop cancers and other health problems. The recall affects various CPAP and Bi-Level PAP devices, and affected consumers are advised to register their devices and review information provided by Philips.
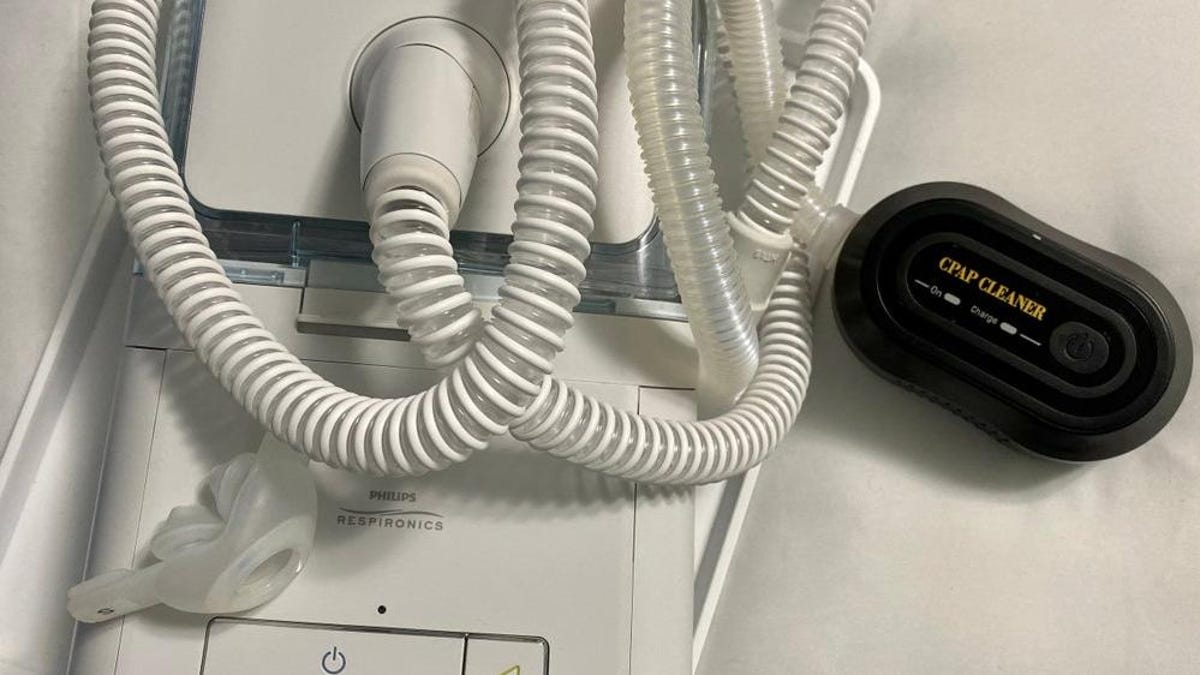
"Philips Ceases US Sales of Sleep Apnea Machines Due to Safety Concerns"
Philips has halted sales of its sleep apnea machines in the US following a global recall due to concerns that the devices could release harmful particles and chemicals into users' lungs. The company initially promised to repair the machines, but the process dragged on for years, leaving patients with dangerous machines or untreated sleep problems. Philips has reached a tentative deal with the US FDA to stop sales of its Respironics devices in the US, estimating the process may cost upwards of $400 million. The consent decree lays out guidelines for the company to meet before bringing the machines back to the US market, while plans to continue the sleep apnea business in other parts of the world are subject to certain requirements.

"Philips Faces $400M Loss as CPAP Sales Halted Over Safety Concerns"
Philips has agreed to halt sales of its CPAP devices in the US under a tentative agreement with regulators, following a global recall of over 5 million machines due to potential health risks from degraded foam. The company could face a $400 million cost as it works to address the issue and meet corrective actions outlined by the FDA. While it will continue servicing previously sold machines, it cannot sell new ones until compliance is achieved. The FDA has warned of potential health risks associated with the foam degradation, and the company still faces personal injury lawsuits related to the devices.
