"New Drug Offers Hope for Sleep Apnea Patients Beyond CPAP"
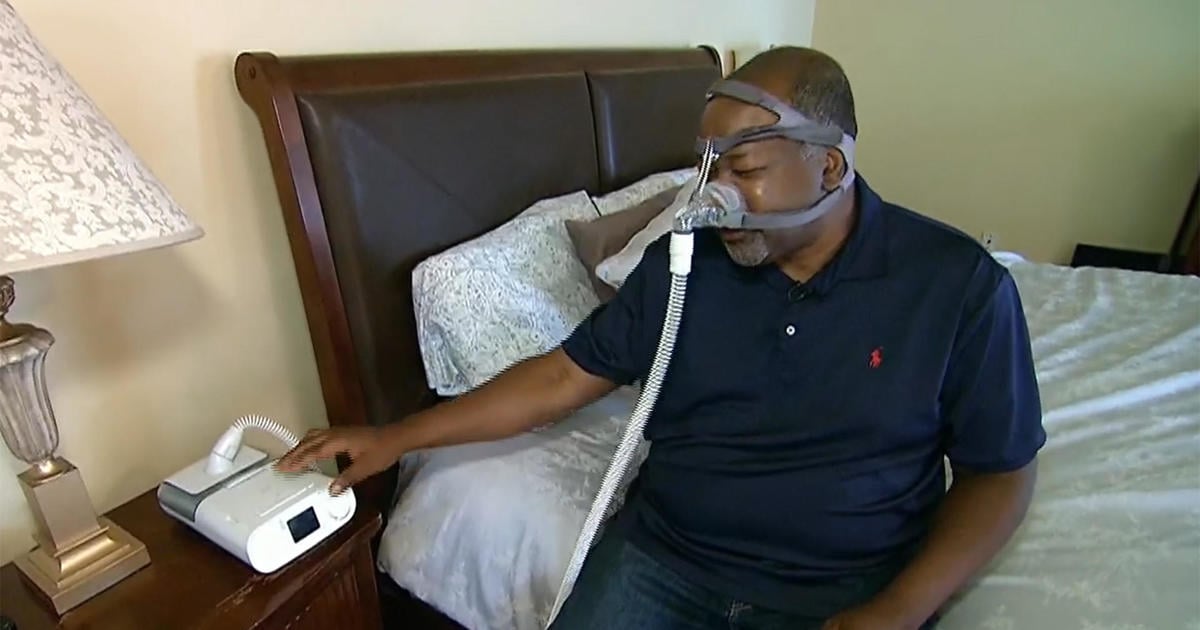
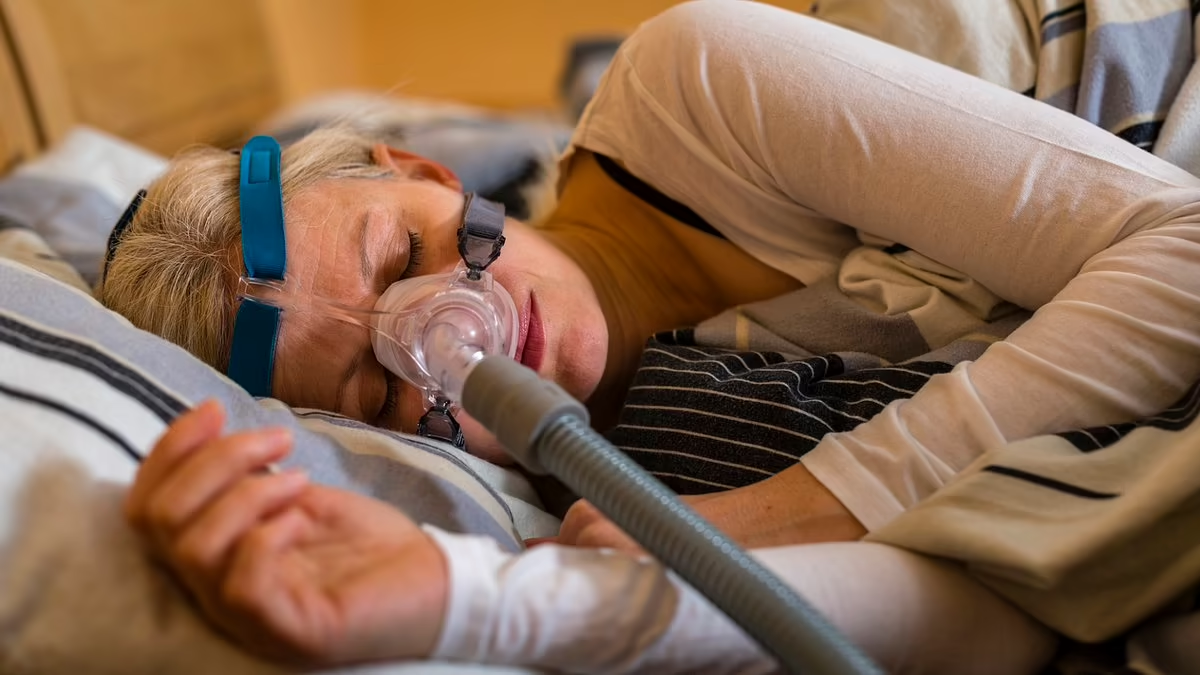
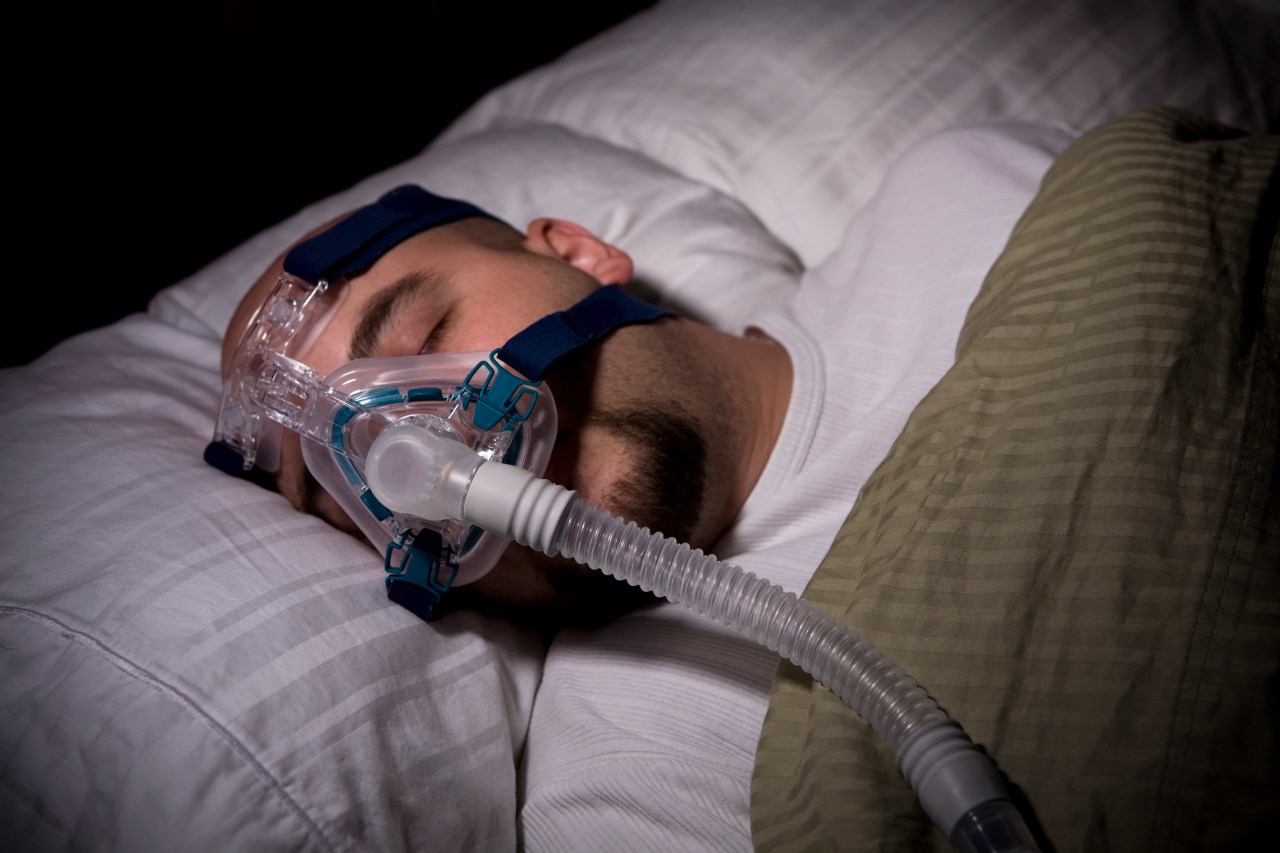
Researchers are conducting a third clinical trial for AD109, a drug that could potentially replace CPAP machines for people with mild obstructive sleep apnea. Developed by Apnimed, AD109 combines aroxybutynin and atomoxetine, and has shown promising results in improving sleep apnea symptoms. With 39 million Americans affected by sleep apnea, this drug could offer a more convenient treatment option.