"Philips Ceases US Sales of Sleep Apnea Machines Due to Safety Concerns"
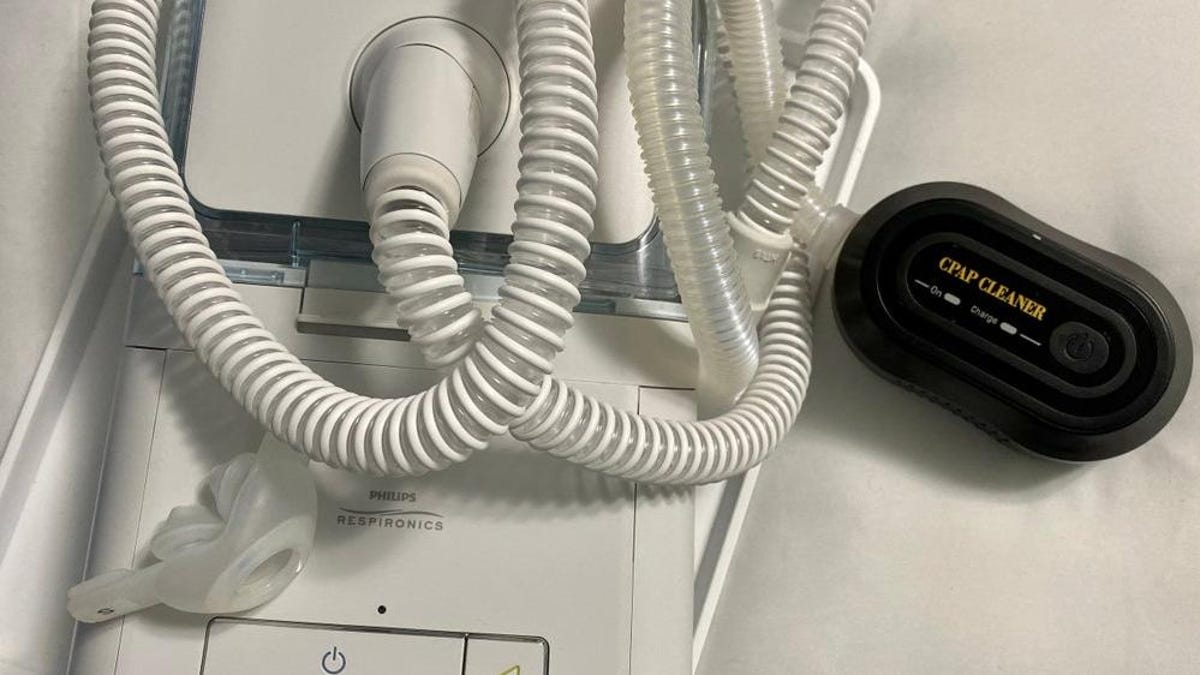
Philips has halted sales of its sleep apnea machines in the US following a global recall due to concerns that the devices could release harmful particles and chemicals into users' lungs. The company initially promised to repair the machines, but the process dragged on for years, leaving patients with dangerous machines or untreated sleep problems. Philips has reached a tentative deal with the US FDA to stop sales of its Respironics devices in the US, estimating the process may cost upwards of $400 million. The consent decree lays out guidelines for the company to meet before bringing the machines back to the US market, while plans to continue the sleep apnea business in other parts of the world are subject to certain requirements.
- Philips Halts Sales of Sleep Apnea Machines After Pumping Dangerous Foam Into People's Lungs Gizmodo
- Philips Suspends Sales of CPAP and Other Breathing Devices After Recall The New York Times
- Philips announces its 2023 Fourth-Quarter and Annual Results Philips
- Philips to halt sales of sleep apnea machines in US over ongoing safety concerns USA TODAY
- Amid Recall Crisis, Philips Agrees to Stop Selling Sleep Apnea Machines in the United States ProPublica
Reading Insights
0
1
1 min
vs 2 min read
68%
387 → 123 words
Want the full story? Read the original article
Read on Gizmodo