Philips to Acquire SpectraWAVE to Enhance AI Coronary Imaging
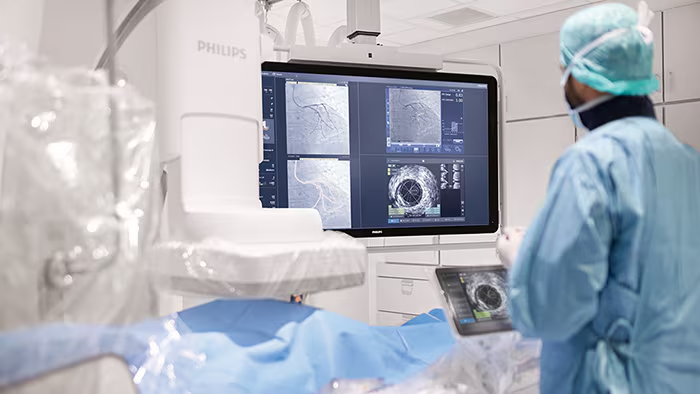
Philips is acquiring SpectraWAVE to enhance its portfolio of AI-powered coronary intravascular imaging and physiological assessment technologies, including HyperVue Imaging System and X1-FFR, aiming to improve outcomes in coronary artery disease treatment through advanced, integrated, and AI-supported solutions.