"FDA Expands CRISPR Gene-Editing Approval for Beta Thalassemia and Second Disease"
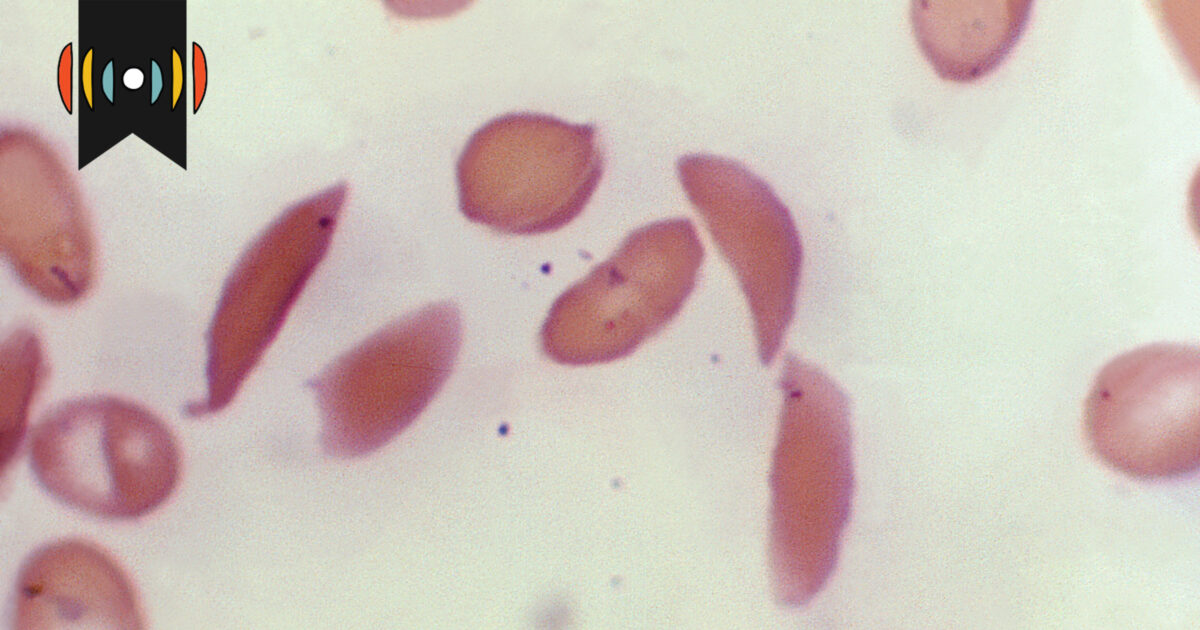
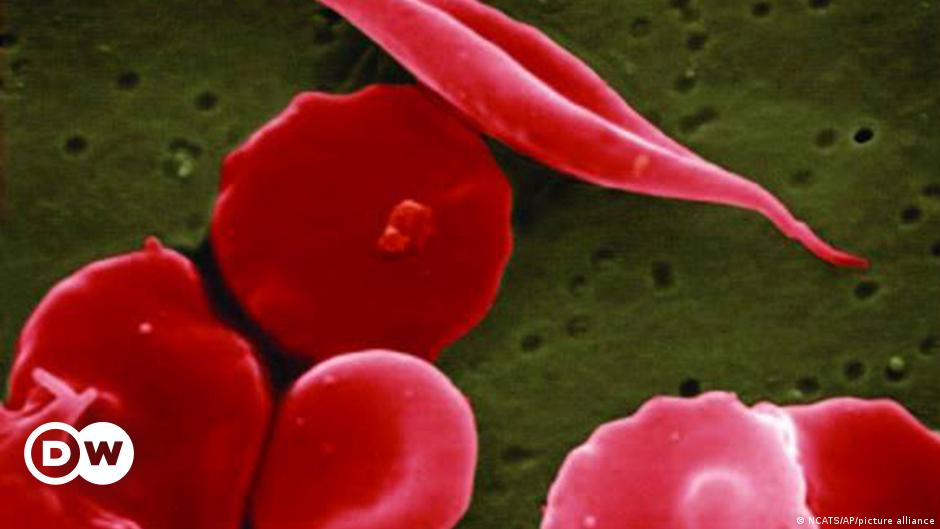
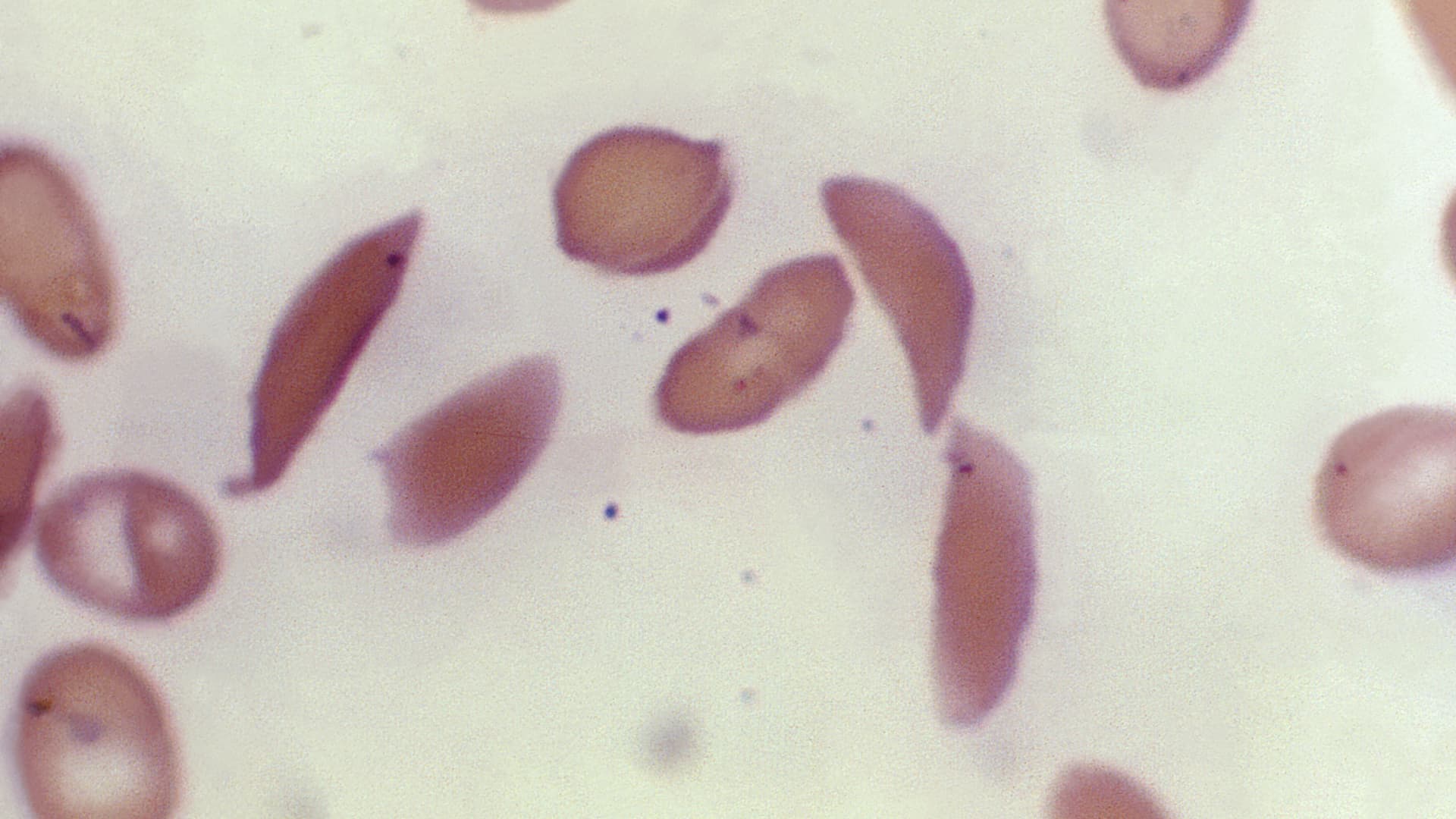
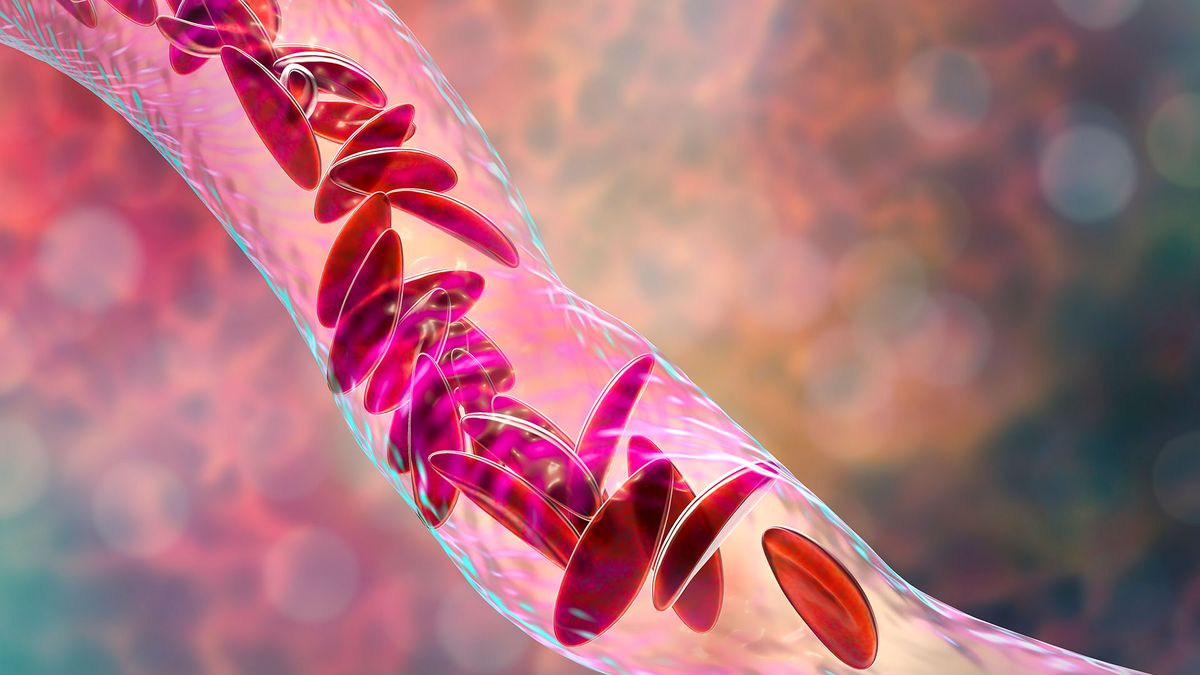
The FDA has expanded its approval of the CRISPR-based medicine Casgevy to include the treatment of beta thalassemia, an inherited blood disorder, following its initial approval for sickle cell disease in December. Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, the therapy's expanded approval comes ahead of the FDA's decision deadline and is seen as a promising development for patients with thalassemia.