"Revolutionary CRISPR Gene-Editing Breakthrough Offers New Hope for Sickle Cell Treatment"
TL;DR Summary
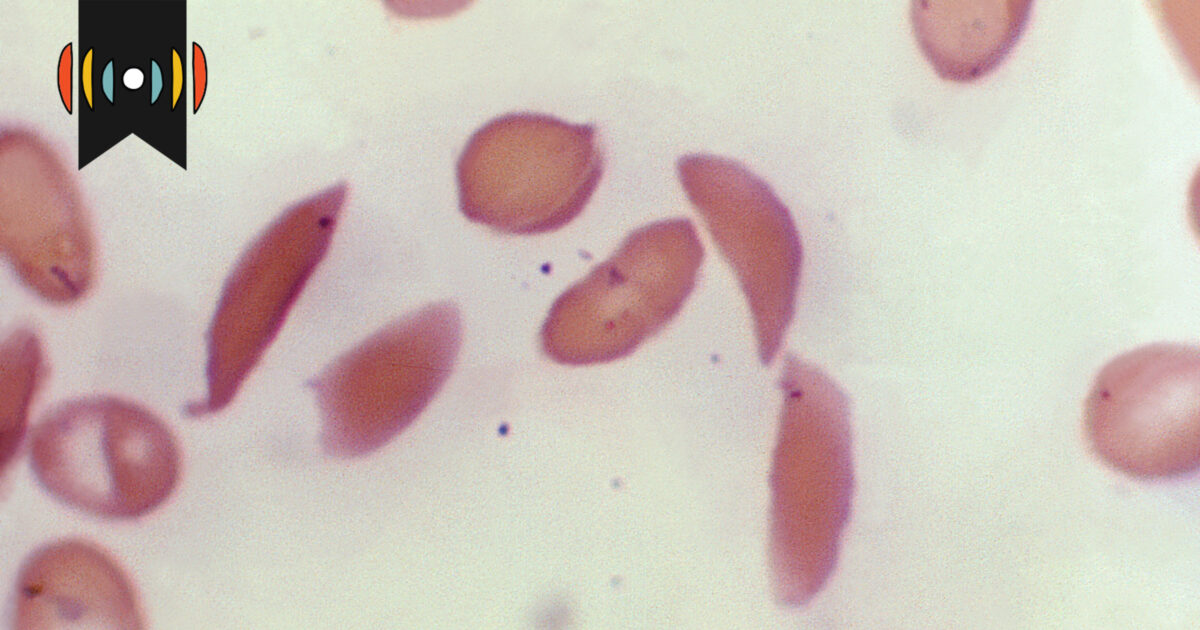
The FDA has approved a new gene-editing treatment called Casgevy for sickle cell disease, which uses CRISPR technology to edit DNA in adult stem cells. This treatment works by turning off an inhibitor of fetal hemoglobin production, allowing the body to produce hemoglobin that can effectively carry oxygen, unlike the mutated form in sickle cell patients. The approval marks a significant advancement in gene therapy, offering a potential cure for sickle cell disease while adhering to ethical standards by only affecting the treated individual and not future generations.
- FDA approves gene-editing treatment for sickle cell disease WORLD News Group
- Sickle cell patients see hope in revolutionary gene therapy Chicago Tribune
- Boston-area companies usher in 'the age of genomic medicines' - Boston Business Journal The Business Journals
- CRISPR Gene Editing Had a Breakthrough Year—and It's Only Getting Started Singularity Hub
- A breakthrough has been announced in Sickle Cell treatment WSB Atlanta
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
6 min
vs 7 min read
Condensed
93%
1,286 → 88 words
Want the full story? Read the original article
Read on WORLD News Group