"Breakthrough Gene-Editing Treatment Offers Hope for Sickle Cell Disease Patients"
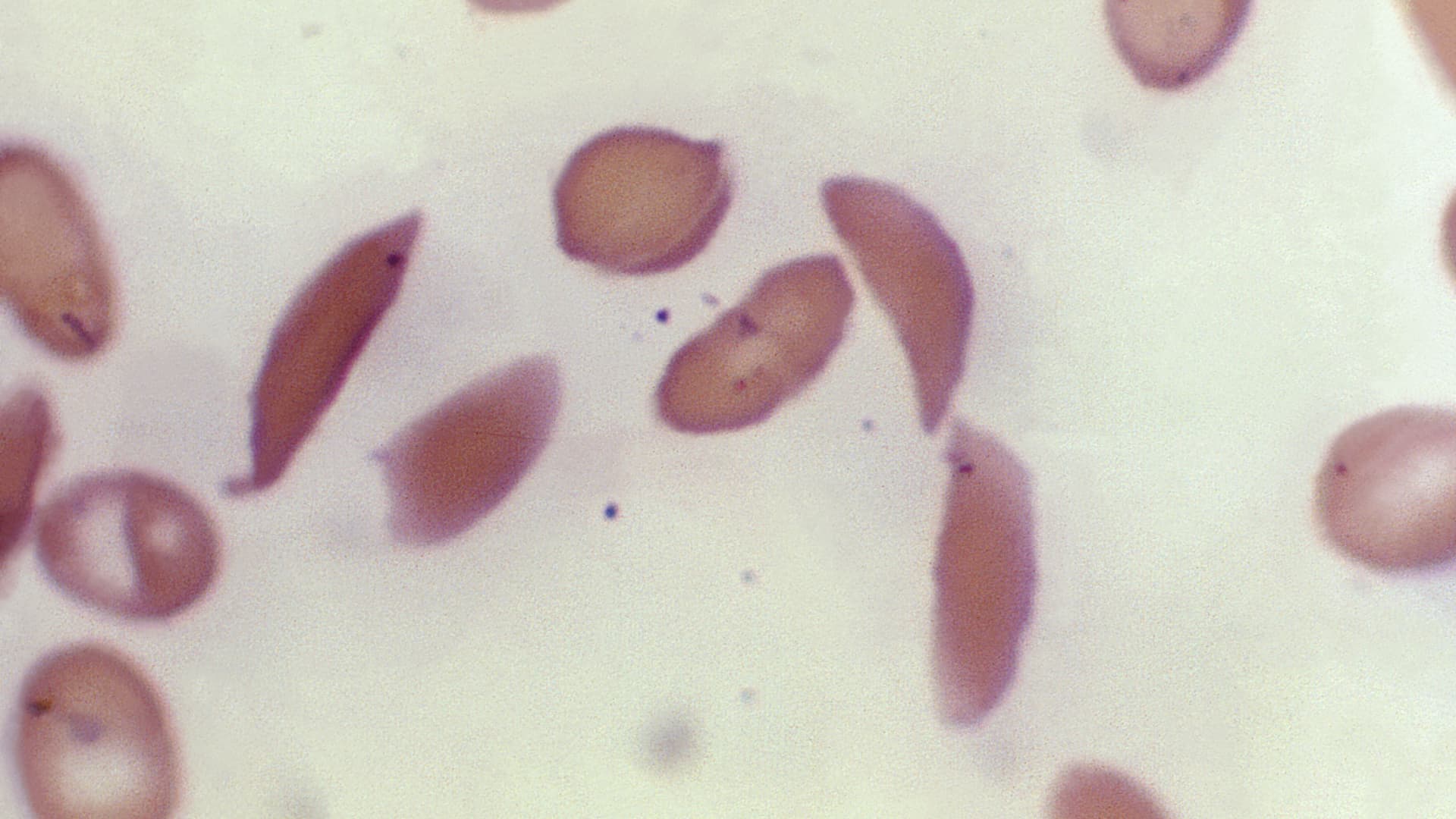
The U.S. Food and Drug Administration has approved the first gene-editing treatment, Casgevy, for sickle cell disease. Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy uses CRISPR technology to edit a person's genes and turn on fetal hemoglobin, helping red blood cells maintain their healthy shape. Clinical trials have shown that Casgevy eliminates pain crises in most patients. However, challenges remain in reaching the thousands of people who could benefit from the treatment, including potential cost barriers and the complexity of administering the therapy. The FDA has also approved a separate gene therapy by Bluebird Bio, called Lyfgenia, for the treatment of sickle cell disease.
- U.S. approves first gene-editing treatment, Casgevy, for sickle cell disease CNBC
- FDA expected to approve first CRISPR gene-editing treatment, bringing hope to thousands with sickle cell disease CNN
- The Download: inside the first CRISPR treatment, and smarter robots MIT Technology Review
- Readout Newsletter: CRISPR with Casgevy, Atlas Venture, etc STAT
- Mayo Clinic Minute: Sickle cell disease explained Mayo Clinic
Reading Insights
0
1
2 min
vs 3 min read
82%
598 → 105 words
Want the full story? Read the original article
Read on CNBC