FDA Approves CRISPR Gene-Editing for Sickle Cell Disease Treatment
TL;DR Summary
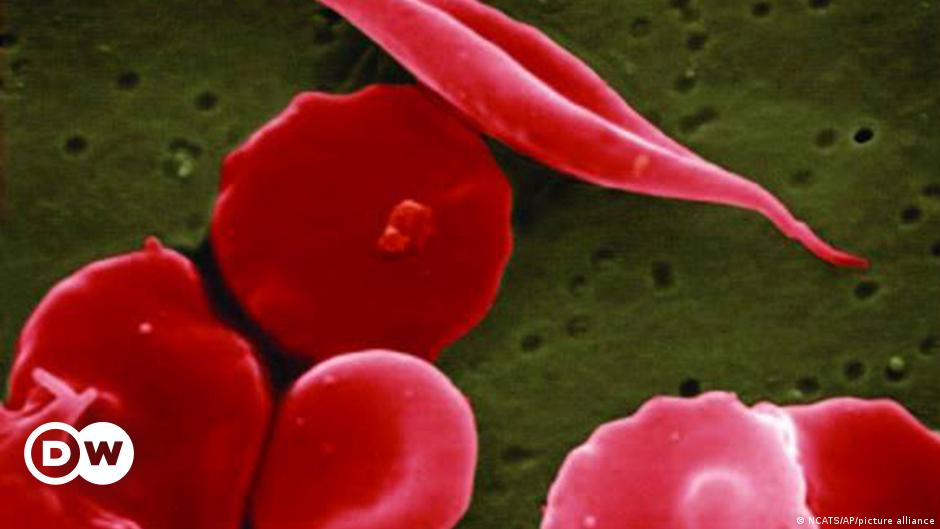
The US Food and Drug Administration (FDA) has approved two groundbreaking gene-editing treatments, Casgevy and Lyfgenia, for sickle cell disease. Casgevy, based on the CRISPR gene editing tool, targets the problematic gene in a patient's bone marrow stem cells, resulting in the production of properly functioning hemoglobin. Lyfgenia utilizes a harmless virus to insert a gene into patients' stem cells. Sickle cell disease is a rare and life-threatening blood disorder that primarily affects people of African or Caribbean descent. The approval of these treatments offers hope for individuals with sickle cell disease, who previously had limited treatment options.
- US approves CRISPR gene-editing to treat sickle cell disease DW (English)
- F.D.A. Approves 2 Sickle Cell Treatments, One Using CRISPR Gene Editing The New York Times
- FDA approves groundbreaking sickle cell anemia treatment NBC News
- A Sickle Cell Breakthrough Is Here. Now the Hard Part. Bloomberg
- Ask science reporter Carolyn Y. Johnson about CRISPR gene-editing The Washington Post
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
1 min
vs 2 min read
Condensed
75%
395 → 98 words
Want the full story? Read the original article
Read on DW (English)