Revolutionary Cancer Treatments: Sound Waves, AI, and Precision Medicine
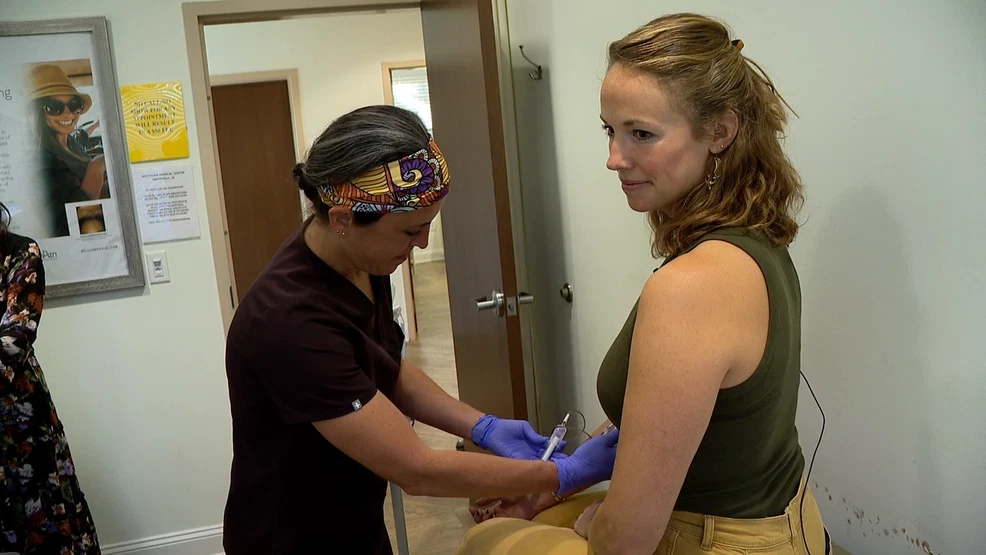
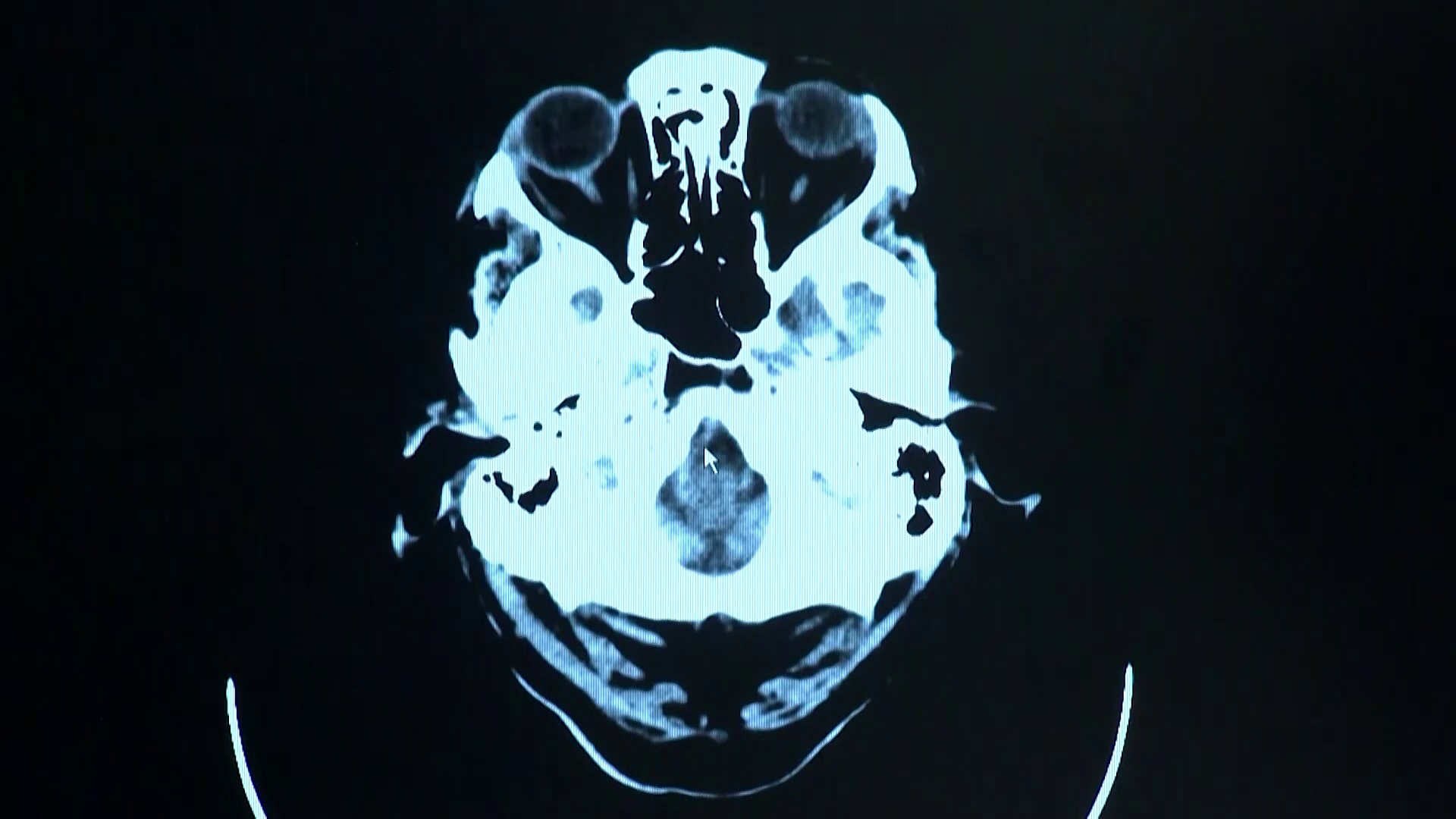
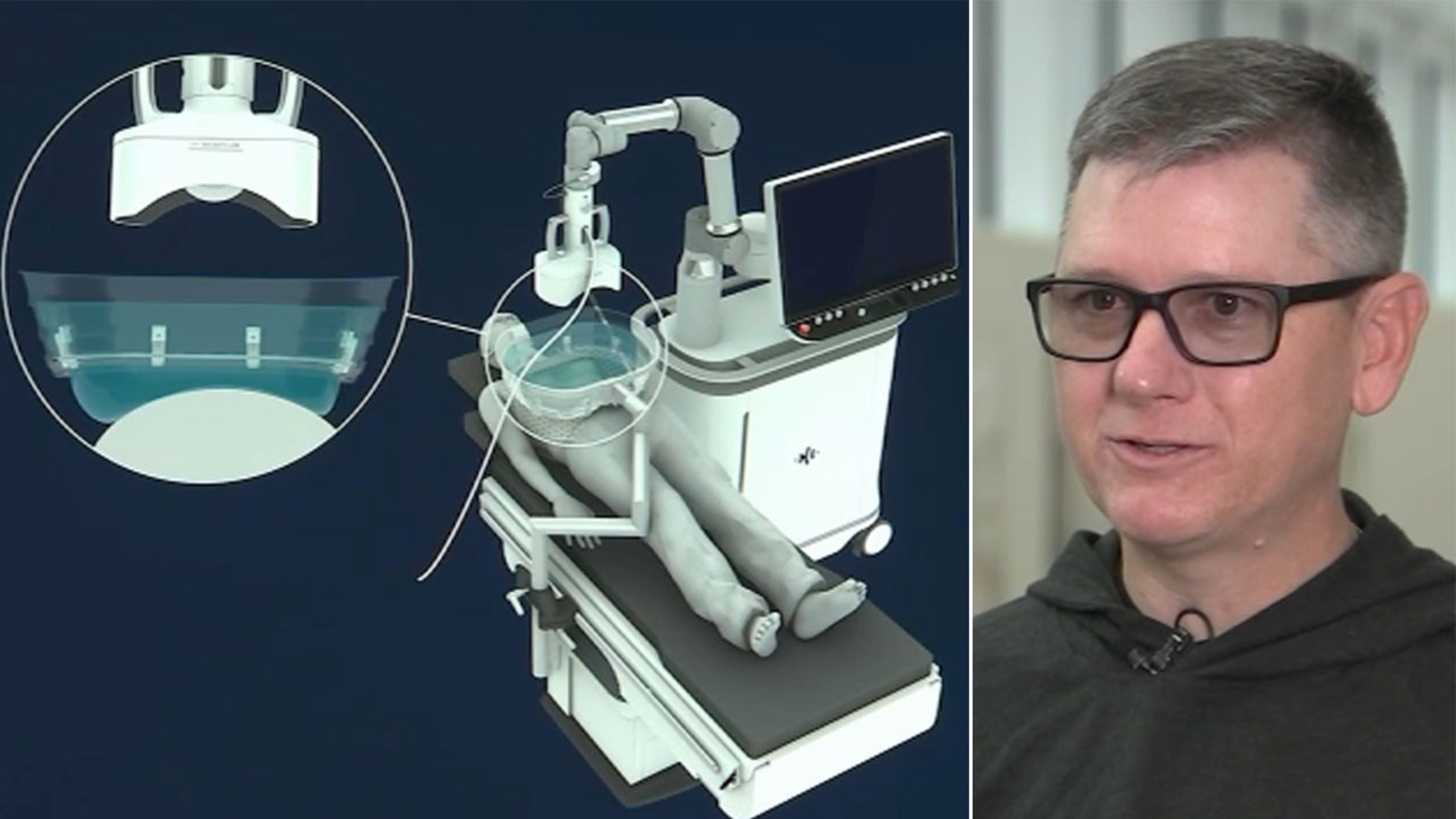
Histotripsy, a new FDA-approved cancer treatment, uses focused ultrasound waves to destroy tumors without harming surrounding tissue. This innovative method, which involves a water medium to focus soundwaves, offers advantages over traditional treatments like radiation. Chris Donaldson, a patient with ocular melanoma that spread to his liver, experienced successful results with histotripsy, leaving his liver cancer-free. The treatment, developed over 20 years, shows promise for other cancers and may enhance the body's defenses by leaving benign genetic material.