Menopause's Unexpected Impact on Eye Health
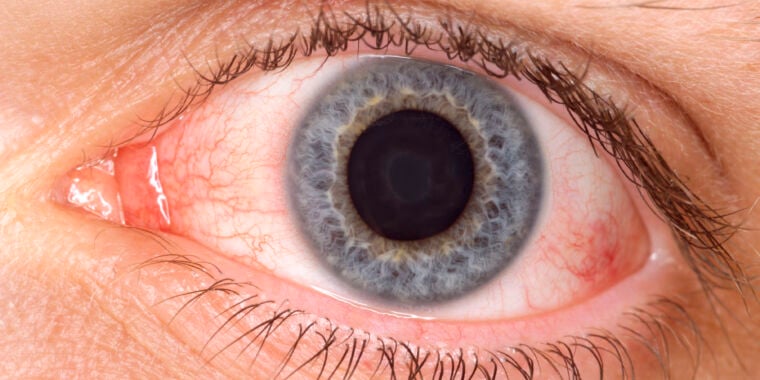
Menopause can affect women's eyesight, particularly causing dry eyes and irritation, which are often overlooked. These symptoms may be linked to hormonal changes, and treatments like eye drops, warm compresses, or hormone therapy can help. Women are encouraged to seek regular eye checks and consult a doctor if symptoms persist.