FDA warns against using amniotic fluid as eye drops.
TL;DR Summary
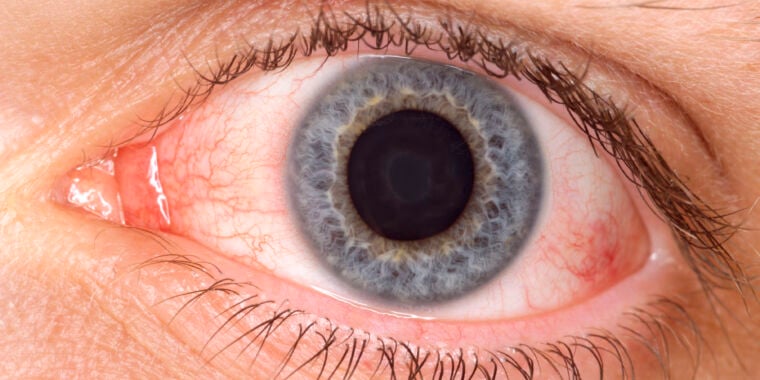
The FDA has issued a public safety notification warning people not to use eye drops containing amniotic fluid, which is largely fetal urine, to treat eye conditions such as dry eyes and inflammation. The drops are unapproved and require an investigational new drug application and full FDA approval before hitting the market. The agency has found unapproved products on the market, raising potential significant safety concerns, and there is only one published clinical trial of amniotic fluid eye drops, which found them to be ineffective.
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
4 min
vs 5 min read
Condensed
91%
928 → 85 words
Want the full story? Read the original article
Read on Ars Technica