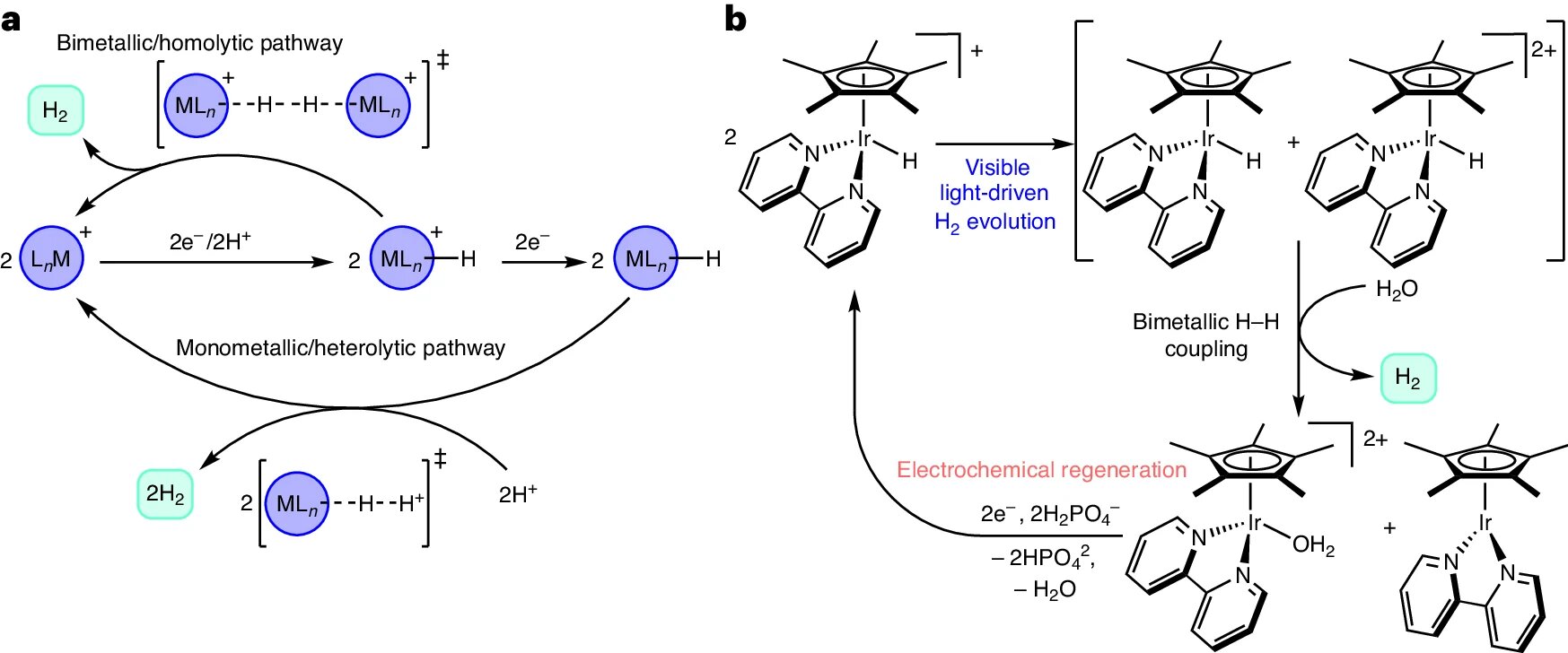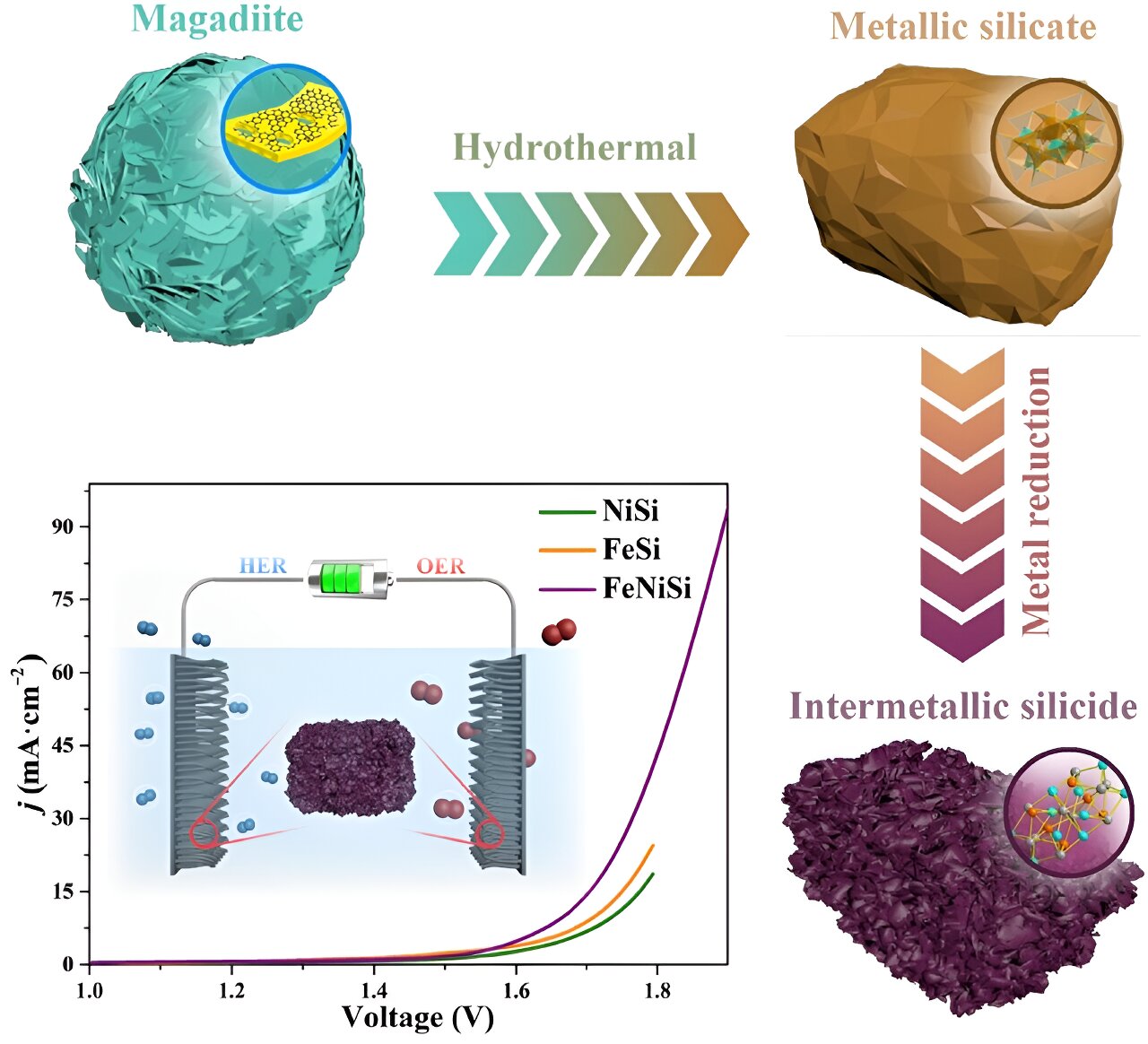
"Nano-Confinement: A Breakthrough in Hydrogen Production"
Researchers at Lawrence Livermore National Laboratory have discovered that nano-confinement can significantly enhance hydrogen production efficiency by altering water reactivity and proton transfer mechanisms. Using advanced molecular dynamics simulations, they found that confining water in nanopores smaller than 0.5 nanometers reduces activation energy for proton transport, suggesting new strategies for improving electrocatalyst performance and durability.