"Philips Respironics Faces Uncertain Future Amid Sleep Apnea Machine Recall and FDA Investigation"
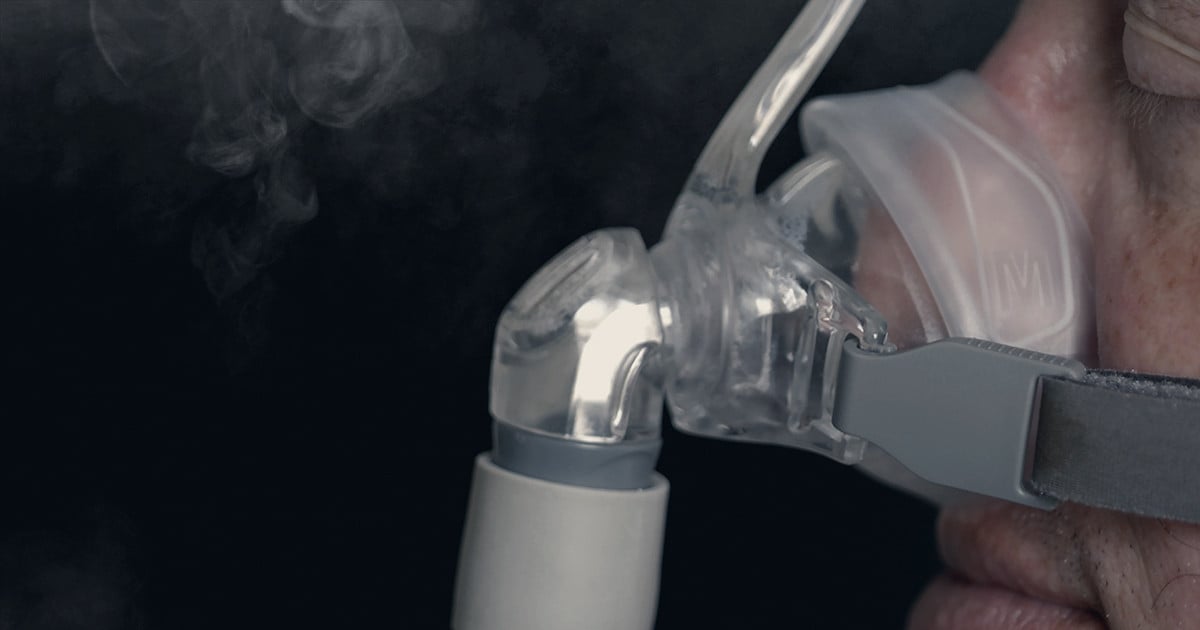
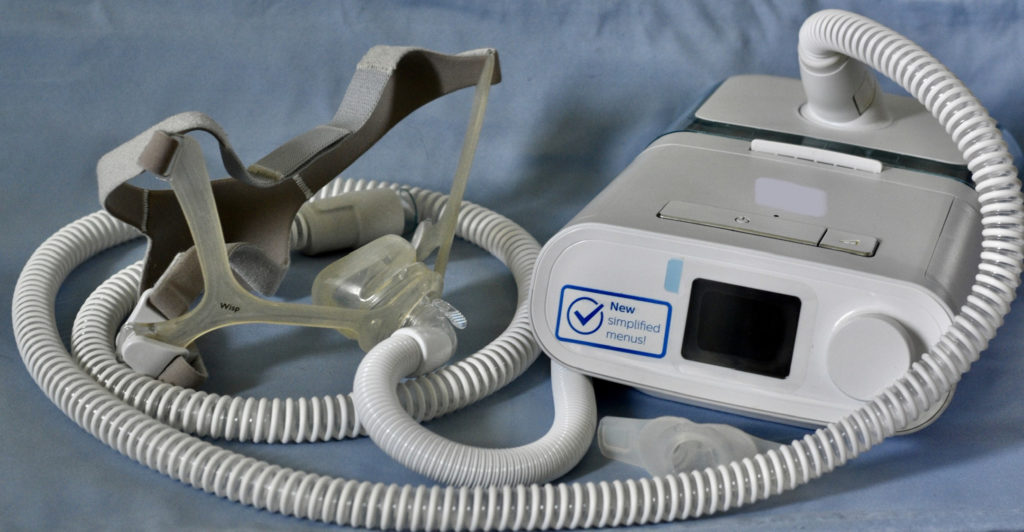
The future of Philips Respironics, a significant contributor to Western Pennsylvania's economy, is uncertain, raising questions about its impact on the region. The company, which specializes in sleep apnea and respiratory care products, has faced challenges such as product recalls and regulatory issues, leading to speculation about potential changes in ownership or operations.