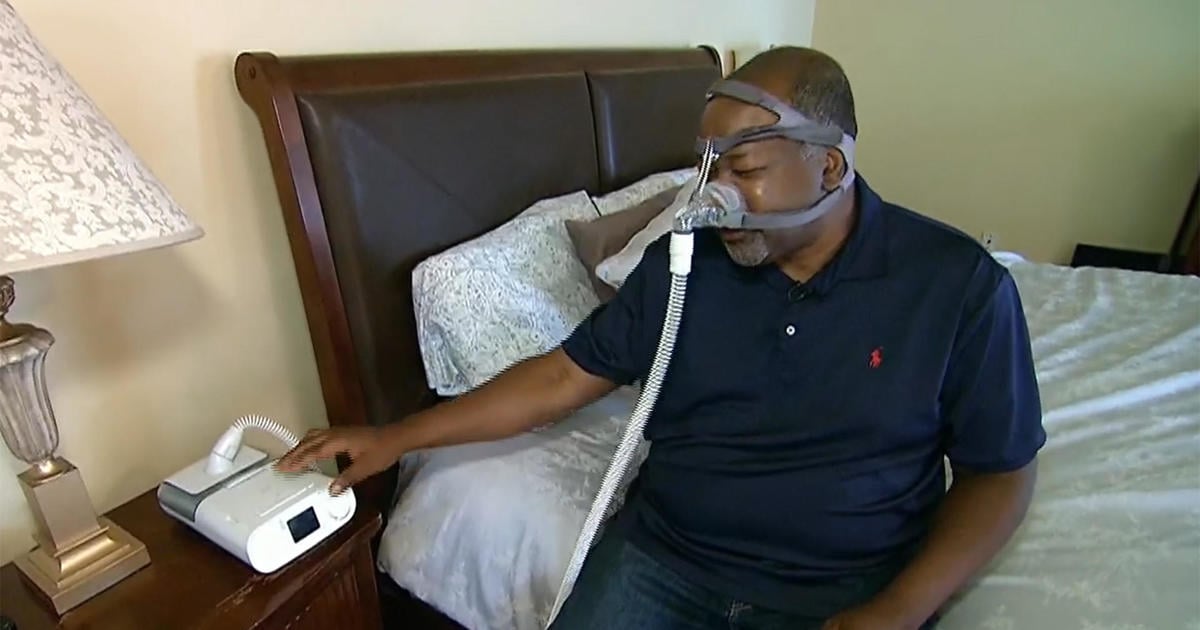
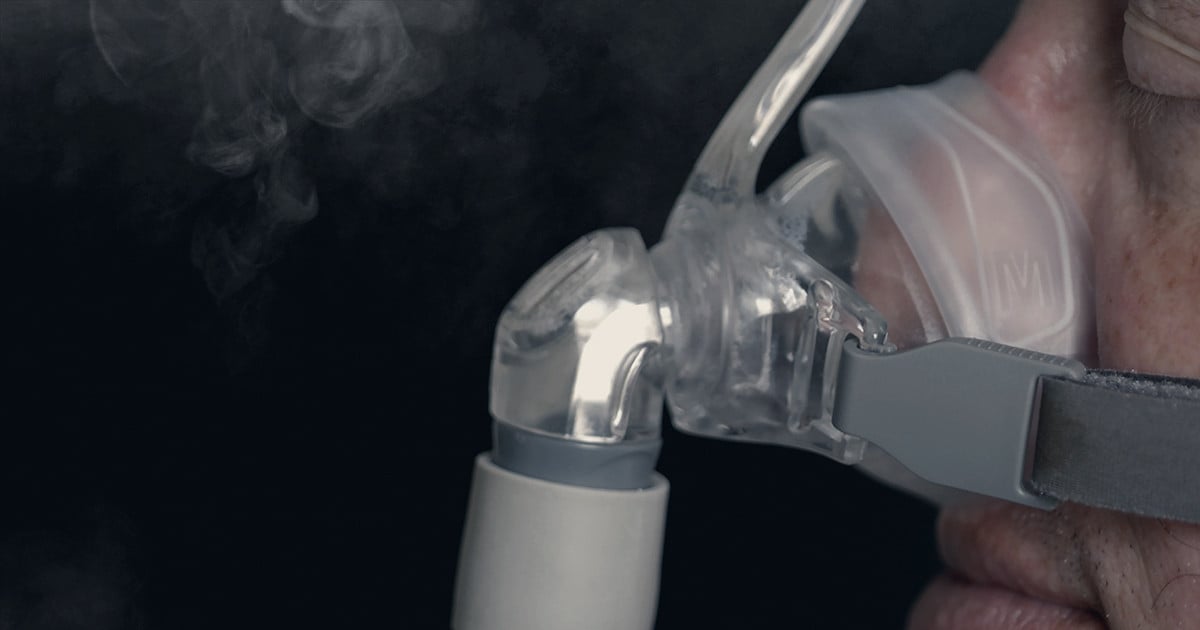
"Philips Ordered to Overhaul Sleep Apnea Device Manufacturing After Recall"
Philips, responsible for a global recall of sleep apnea machines due to defective foam, has been ordered to overhaul its manufacturing and quality control systems by federal officials. The company must also continue to replace, repair, or provide refunds to all U.S. customers affected by the defective devices. The settlement comes after years of delays in addressing the issue, with lawsuits filed by affected individuals in multiple countries. The FDA found instances where Philips was aware of the problem with its foam years before the recall, prompting the agency to order clearer information about the health risks of the products and increased outreach to affected customers.