South Africa's HIV vaccine trial adapts after USAID funding freeze
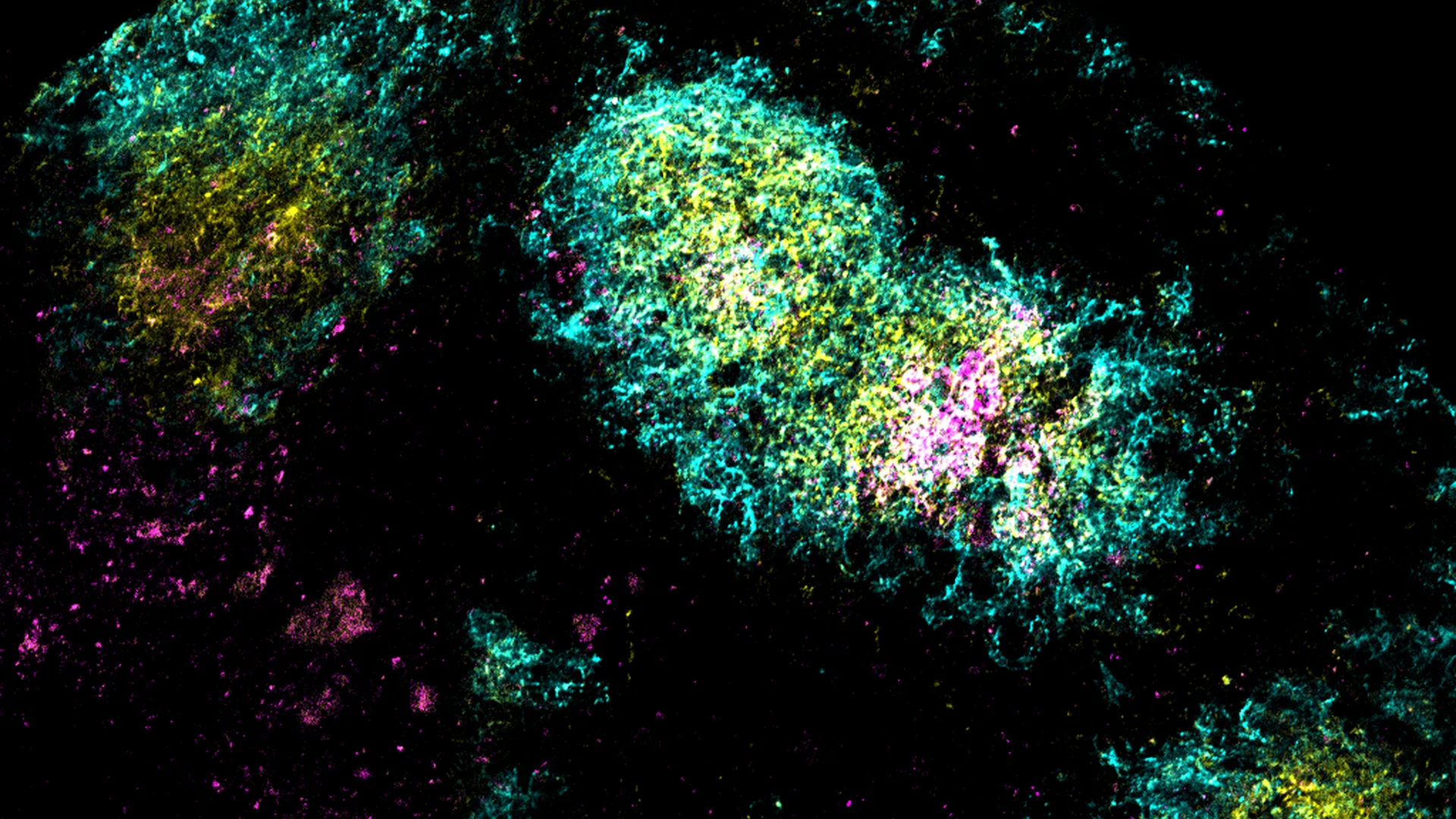
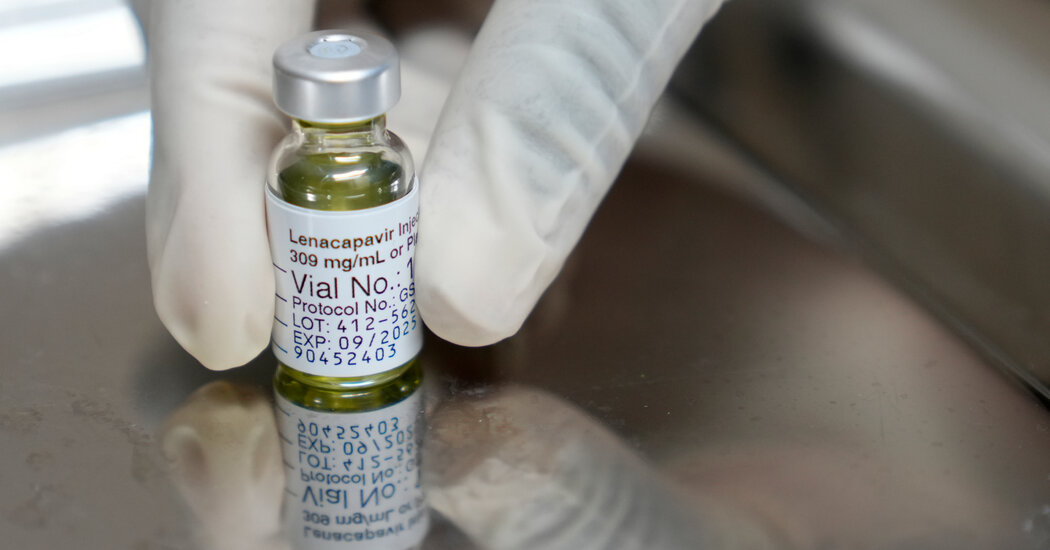
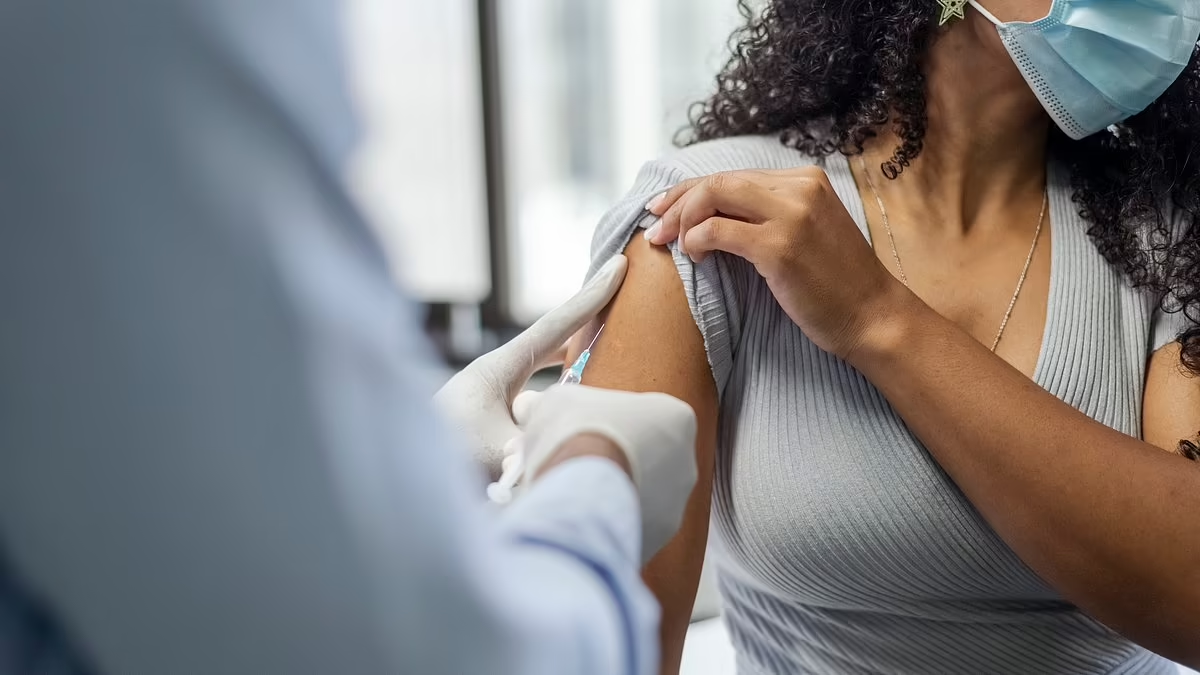
A South African-led HIV vaccine trial faced a funding freeze after USAID cut support, prompting researchers to pivot to local funding sources and scale back the study to a South Africa–based trial in Cape Town, with recruitment underway as scientists pursue a vaccine strategy based on broadly neutralizing antibodies.