FDA Approves Biannual HIV Prevention Drug Lenacapavir
TL;DR Summary
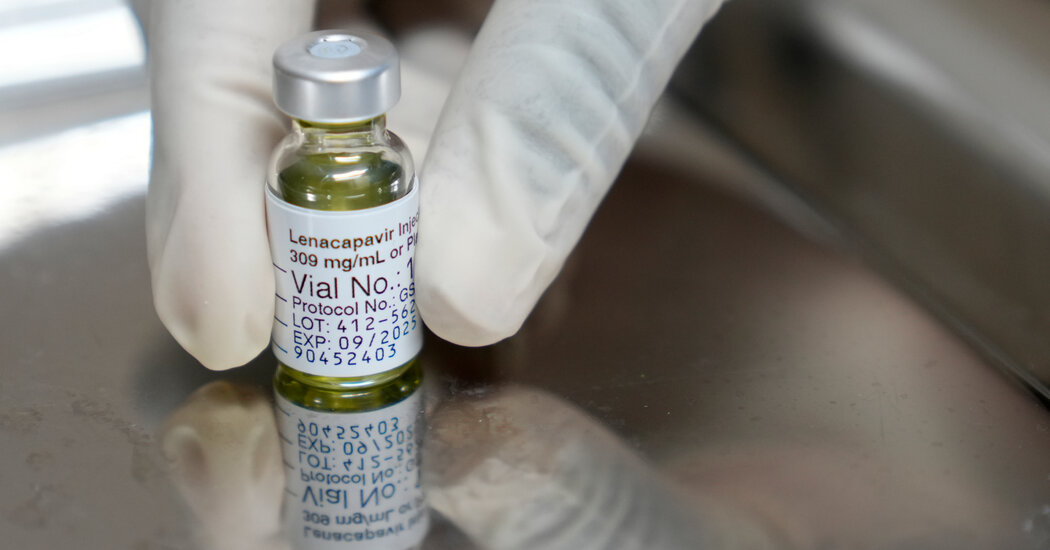
The FDA has approved Lenacapavir, a twice-yearly injection that effectively prevents HIV infection, marking a significant advancement in HIV prevention efforts. However, its rollout in low-income countries is uncertain due to recent cuts in global health funding.
- Regulators Approve Lenacapavir for H.I.V. Prevention The New York Times
- FDA approves new twice-yearly HIV shot. What to know USA Today
- FDA approves powerful HIV prevention drug: What to know about lenacapavir NBC News
- HIV protection with just two shots a year: FDA approves Gilead drug statnews.com
- ‘HIV-ending’ drug could be made for just $25 per patient a year, say researchers The Guardian
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
1 min
vs 1 min read
Condensed
78%
166 → 37 words
Want the full story? Read the original article
Read on The New York Times