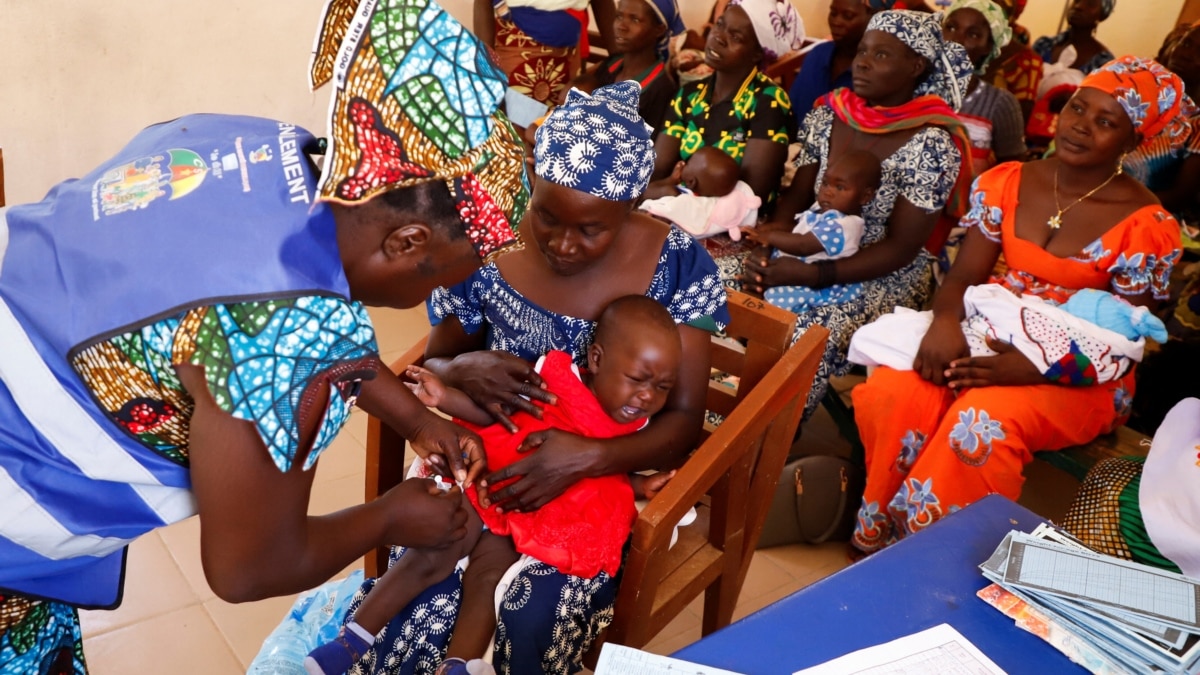
"Cameroon Initiates Groundbreaking Malaria Vaccine Program for Children"
Cameroon has launched the world's first major malaria vaccine program for children, using the RTS.S vaccine developed by GlaxoSmithKline. The vaccine is meant to work with other preventive measures to fight the disease, and the country aims to vaccinate about 250,000 children this year and next. The campaign is seen as a major step in the fight against malaria in Africa, where the disease accounts for about 95 percent of the world's malaria deaths. The World Health Organization has approved two malaria vaccines, with hopes that their use could sharply reduce severe infections and hospitalizations.