FDA approves first-ever RSV vaccine for older adults.
TL;DR Summary
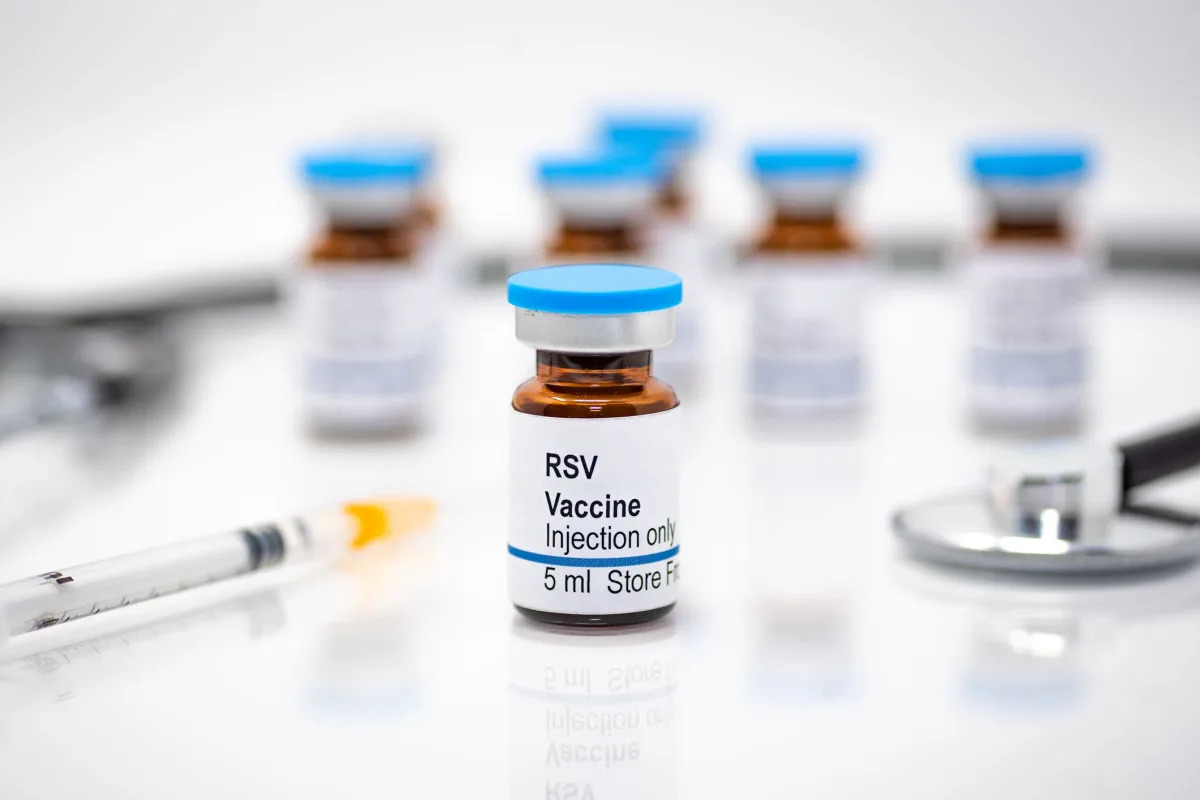
The FDA has approved the first-ever vaccine to combat severe respiratory syncytial virus (RSV), called Arexvy, developed by GlaxoSmithKline, for adults aged 60 and older. The vaccine was 82% effective at preventing lower respiratory tract illness caused by RSV and 94% effective in those with underlying medical conditions. RSV is the leading cause of hospitalizations among newborns and younger children and causes more than 177,000 hospitalizations and 14,000 deaths among older adults each year. Other RSV vaccines and treatments are also under review by regulatory agencies.
- FDA approves first-ever RSV vaccine, shots to be available for older adults Yahoo! Voices
- Boston doctor discusses FDA approval of first RSV vaccine WCVB Channel 5 Boston
- FDA approves first vaccine for RSV, a moment six decades in the making CNN
- What is Arexvy? A look at the new, FDA-approved RSV vaccine WGN News
- Live news updates from May 3: Fed raises rates 25bps, Russia accuses Ukraine of Putin assassination attempt Financial Times
Reading Insights
Total Reads
0
Unique Readers
6
Time Saved
2 min
vs 3 min read
Condensed
84%
528 → 86 words
Want the full story? Read the original article
Read on Yahoo! Voices