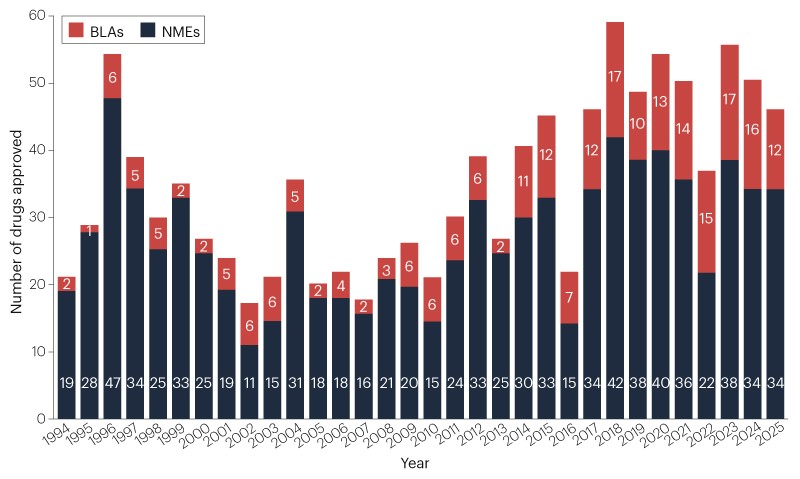
FDA Adopts One-Pivotal-Trial Path for Drug Approvals
The FDA will default to approving drugs based on a single well-controlled pivotal trial, while allowing supporting confirmatory evidence such as mechanistic data, related indications, animal models, or real-world data. The move, framed by FDA leaders as a shift from an outdated ‘two-trial dogma,’ aims to speed development and cut costs, but has drawn criticism from some policymakers and former FDA officials who warn it could weaken patient protections. The policy preserves discretion to require additional studies in certain cases, and reactions are mixed among analysts and industry figures, with Pazdur expressing concerns before his retirement.