2025 FDA Approvals and Their Impact on Biopharma and Ophthalmology
TL;DR Summary
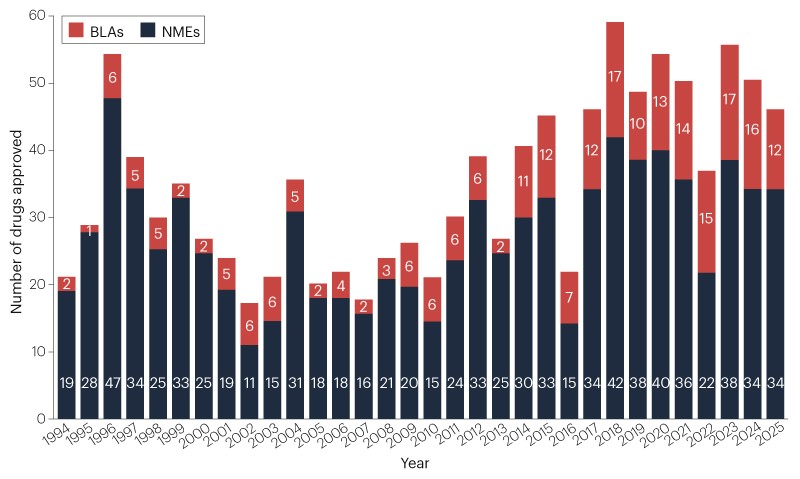
In 2025, the FDA approved 46 new therapeutic agents, including a diverse range of modalities such as biologics, kinase inhibitors, and gene therapies, with cancer remaining the most common therapeutic area. The year was marked by policy changes, staffing challenges, and the launch of new approval pathways, reflecting a tumultuous but innovative period for drug development.
- 2025 FDA approvals Nature
- A Real Test for New FDA Reforms: News Article Independent Institute
- NeurologyLive® Friday 5 — January 2, 2026 | NeurologyLive - Clinical Neurology News and Neurology Expert Insights NeurologyLive
- FDA approvals in 2025: What changed and why it matters for ophthalmologists Ophthalmology Times
- Year in Review: How FDA Guidances Defined the 2025 Biopharma Landscape BioPharm International
Reading Insights
Total Reads
0
Unique Readers
7
Time Saved
20 min
vs 21 min read
Condensed
99%
4,022 → 56 words
Want the full story? Read the original article
Read on Nature