"FDA's 2023 Surge in Drug Approvals Marks a Record Year for Pharmaceuticals"
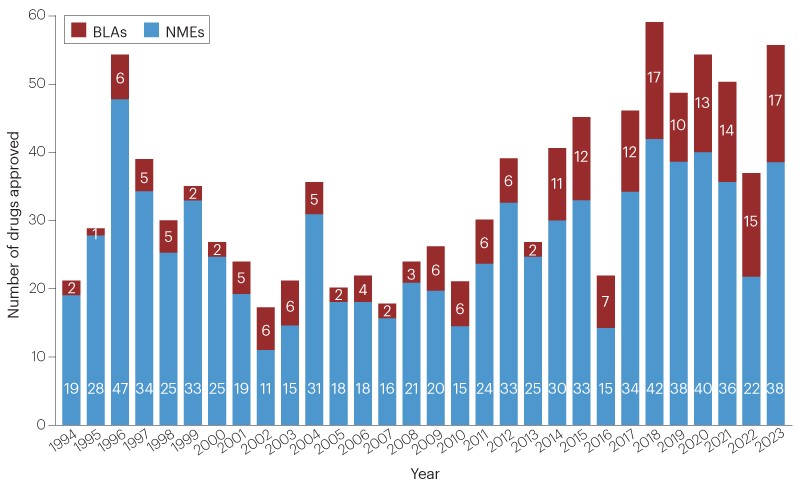
The FDA approved 55 new drugs in 2023, marking a significant increase from the previous year and setting a 10-year rolling average high. Oncology led with 24% of the approvals, followed by neurology. Notable approvals included the first CRISPR–Cas9-based gene editing product, two RSV vaccines, and a burst of gene therapies. AstraZeneca's capivasertib became the first AKT inhibitor approved for breast cancer, and several first-in-class cancer therapies were greenlit. In neurology, Biogen and Ionis's tofersen received accelerated approval for SOD1-mutated ALS, and Eisai and Biogen's lecanemab was approved for Alzheimer's disease. The approvals spanned various modalities, including nucleotide-based therapeutics and T-cell engaging bispecific antibodies.
- 2023 FDA approvals Nature.com
- 2023 drug approvals: After a down year, FDA signs off on a bounty of new meds, including 7 from Pfizer FiercePharma
- FDA's new drug approvals for 2023 rise 51% from last year (NYSE:PFE) Seeking Alpha
- What New Cancer Drugs Were Approved in 2023? www.oncnursingnews.com/
- 2023: The Year in Infectious Disease Contagionlive.com
Reading Insights
0
0
19 min
vs 20 min read
97%
3,988 → 104 words
Want the full story? Read the original article
Read on Nature.com