Sanofi and AstraZeneca's RSV Antibody Faces Limited Supply Amid High Demand
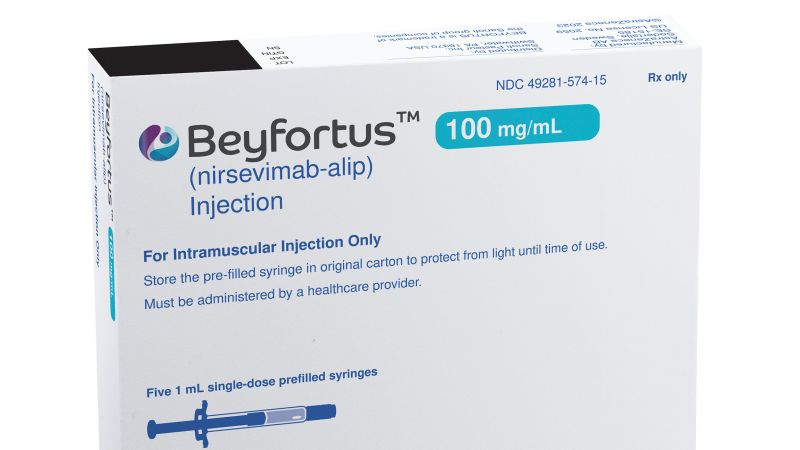
Sanofi anticipates limited supply of its infant RSV antibody Beyfortus, co-developed with AstraZeneca, in the first quarter of this year due to overwhelming demand, with plans to boost production but uncertainty about the extent of progress that can be made in 2024. CEO Paul Hudson highlighted efforts to address manufacturing bottlenecks and increase capacity to tackle supply constraints.