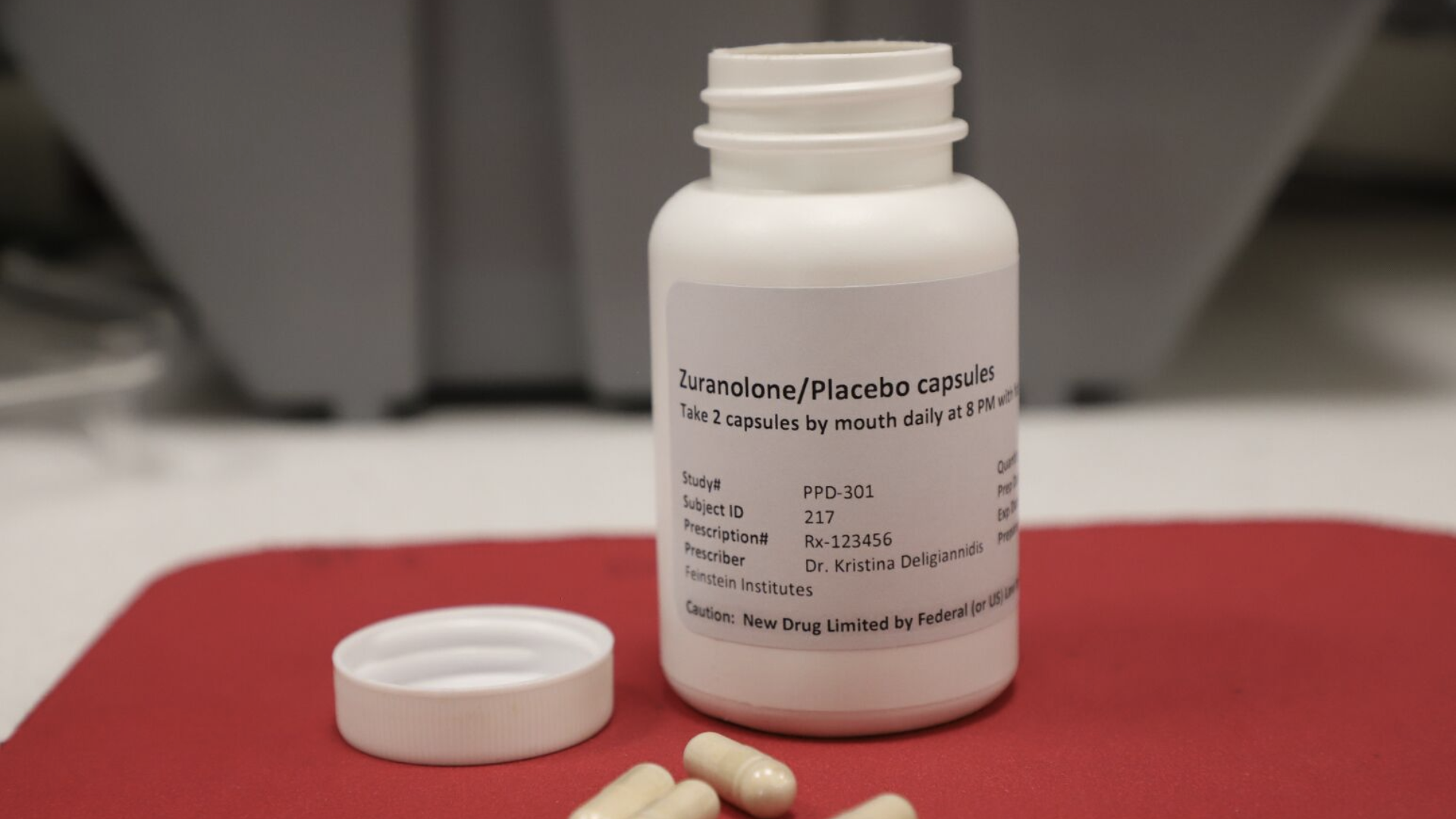
"First Pill for Postpartum Depression Now Available and Effective"
Zuranolone, the first pill for postpartum depression, has been approved and is now being prescribed by psychiatrists. This condition, affecting up to 1 in 5 women, can lead to severe consequences like suicide and developmental delays in infants. Previously, treatment options were limited to intravenous injections with significant side effects. Early reports indicate that Zuranolone shows promise, with some patients experiencing improvements within days, though its long-term efficacy remains uncertain.