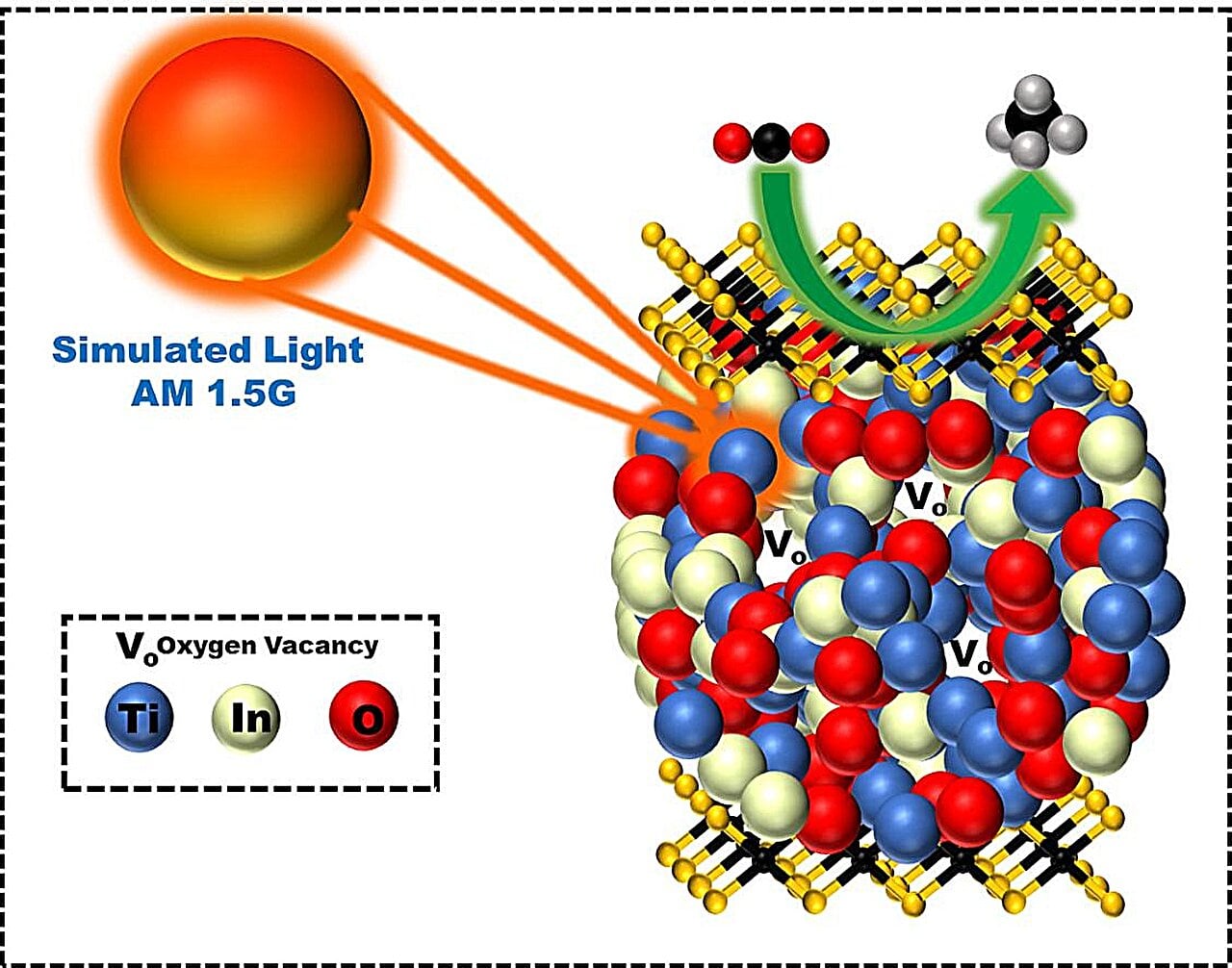
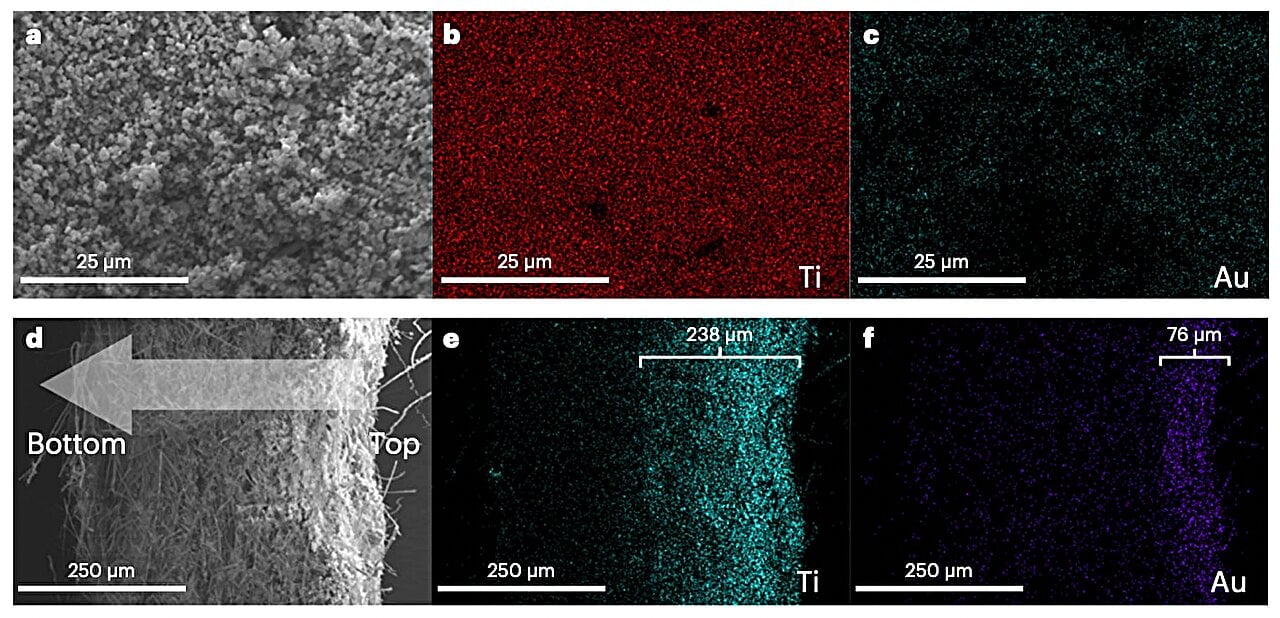
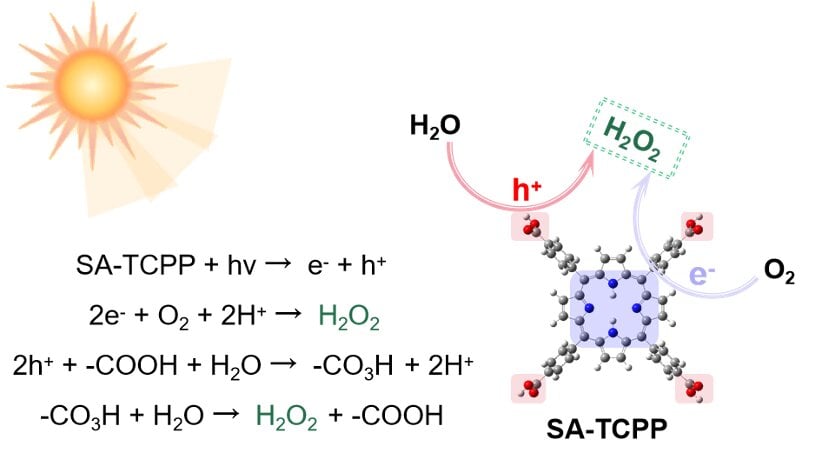
Revolutionary Solar Tech Converts Water to Green Hydrogen
Japanese scientists have developed a new technology using a special photocatalyst to convert sunlight and water into hydrogen fuel, potentially offering a sustainable alternative to fossil fuels. This method, which employs a two-step water-cracking process, has shown promising results in real-world conditions, achieving higher solar energy conversion efficiency than in laboratory settings. However, the current efficiency is still below 5%, and further research is needed to improve photocatalysts and scale up the technology for practical use.