
Gilead's Trodelvy Fails Key Bladder Cancer Trial
Gilead announced that its antibody-drug conjugate Trodelvy failed to meet its primary endpoint in a Phase 3 study for bladder cancer, impacting the company's oncology business.
All articles tagged with #phase 3 study

Gilead announced that its antibody-drug conjugate Trodelvy failed to meet its primary endpoint in a Phase 3 study for bladder cancer, impacting the company's oncology business.

Insmed's stock surged 135% after announcing successful Phase 3 trial results for brensocatib in treating non-cystic fibrosis bronchiectasis, showing significant reductions in pulmonary exacerbations. The company plans to file for FDA approval in late 2024, with potential U.S. launch in mid-2025 and subsequent launches in Europe and Japan.

Eli Lilly's obesity drug Zepbound has shown positive results in treating obstructive sleep apnea (OSA) in a Phase 3 study, potentially positioning it as the first approved treatment for OSA. Patients taking Zepbound experienced a significant reduction in the apnea-hypopnea index compared to those on a placebo, indicating its potential to address breathing interruptions during sleep in individuals with obesity.

Tenax Therapeutics has received FDA clearance for its Investigational New Drug (IND) application for TNX-103, an oral levosimendan, for the treatment of pulmonary hypertension with heart failure with preserved ejection fraction (PH-HFpEF). The company plans to initiate the first of two Phase 3 studies, called the LEVEL Study, in the fourth quarter of 2023. The primary endpoint for both Phase 3 studies will be the six-minute walk distance (6MWD). The FDA does not require a cardiovascular outcomes trial for this indication. TNX-103 has the potential to address the underlying pathophysiology of PH-HFpEF and could provide a new approach for reducing high central and venous blood pressures associated with the condition.
Tenax Therapeutics has received FDA clearance for its Investigational New Drug (IND) application for TNX-103, an oral levosimendan, for the treatment of pulmonary hypertension with heart failure with preserved ejection fraction (PH-HFpEF). The company plans to initiate the first of two Phase 3 studies, called the LEVEL Study, in the fourth quarter of 2023. The Phase 3 program aims to enroll 300 patients for 6 months and 100 patients for 1 year, with the primary endpoint being the 6-minute walk distance. The FDA does not require a cardiovascular outcomes trial, and the use of oral levosimendan in PH-HFpEF is protected by a patent until 2040. PH-HFpEF currently has no FDA-approved treatments, and the prevalence is estimated to exceed 2 million patients in North America by 2030.
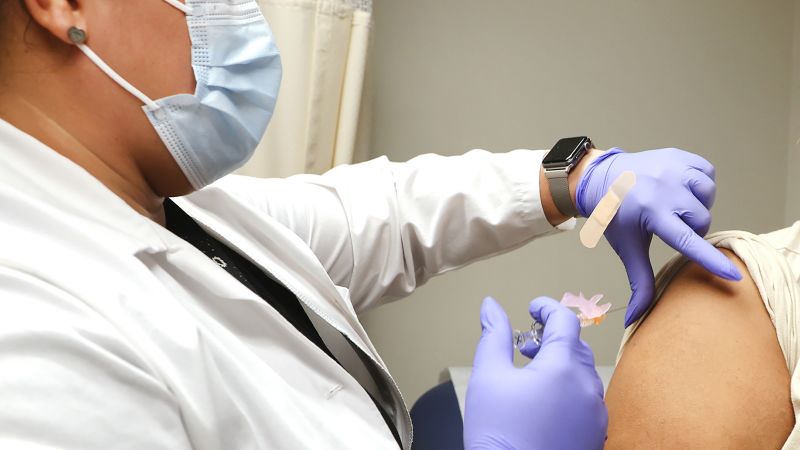
Moderna's experimental mRNA-based seasonal flu vaccine, mRNA-1010, has shown a stronger immune response against four strains of the flu virus compared to a currently marketed vaccine in a Phase 3 study. The positive results pave the way for Moderna to discuss a path to approval with regulators. The company is racing against Pfizer to bring mRNA technology to seasonal flu vaccines and plans to combine Covid-19 mRNA shots with flu and RSV vaccines in the future. Moderna expects a decision from the FDA on its mRNA vaccine for RSV in older adults by April. The safety findings of the flu vaccine study were similar to previous ones, with muscle pain, headache, fatigue, pain, and swelling being the most common reactions. The study focused on the immune response generated by the vaccine rather than its efficacy in protecting against flu disease.
FibroGen's Phase 3 ZEPHYRUS-1 trial evaluating the safety and efficacy of pamrevlumab in patients with idiopathic pulmonary fibrosis (IPF) did not meet the primary endpoint of change from baseline in forced vital capacity (FVC) at week 48. The study compared treatment with pamrevlumab to placebo. Pamrevlumab was generally safe and well tolerated. FibroGen plans to implement a significant cost reduction effort in the U.S. with the intent to extend its cash runway into 2026.